Role of Endoscopic Ultrasonography in Diagnosis, Staging, and Outcome of Gastrointestinal Disease
Using endoscopic ultrasound imaging, highly accurate gastrointestinal tumor characterization and staging is now possible without surgery. Precise endosonographic preoperative staging allows use of the TNM pathological staging system to stratify treatment for esophageal, gastric, rectal, and pancreatobiliary tumors. Endoscopic ultrasonography often establishes a diagnosis in malignant, and in certain patients, benign disease; and characterizes submucosal masses. When histology is required, endoscopic ultrasonography has a critical role in defining an abnormal area for deep submucosal biopsy from the gastrointestinal lumen. Endoscopic ultrasonography is unsurpassed in determining resectability of gastrointestinal neoplasms. It can be used to monitor treatment of downstaged tumors prior to attempted resection. Using endoscopic ultrasonography for earlier diagnosis and precise staging will significantly improve the clinical outcome of patients with gastrointestinal disease as significant advances both in surgical techniques and in combined chemotherapy and radiotherapy continue to be made and applied selectively.
Snady H. Role of endoscopic ultrasonography in diagnosis, staging, and outcome of gastrointestinal disease. The Gastroenterologist 1994; 2:91-110
During the past 10 years, echoendoscopy, or endoscopic ultrasonography (EUS), has proven to be the most significant advance for imaging the gastrointestinal (GI) tract wall and contiguous organs. The detailed resolution seen on EUS images is unmatched by any other current method. Many studies document the high accuracy
of nonoperative GI tumor characterization and staging that is possible with EUS [1]. EUS can provide unique information essential to diagnosing malignancy that is often otherwise obtainable only by surgical techniques. In terms of patient outcome, the role of EUS is clearly woven into the influence of pathological staging strategies and effective treatment on both prognosis and quality of life.
The American joint Committee on Cancer (AJCC) and The International Union Against Cancer (UICC) have each published a manual with recommendations for stage groupings of cancer at all anatomical sites [2,3]. These evolving recommendations are based on contributions from 400 expert participants over 25 years. The AJCC and the UICC, after numerous meetings, reached uniform recommendations to allow the development of I system of staging that would replace other nonuniform methods. This system for staging cancer is now accepted and used worldwide with the hope that "accurate staging of cancer will be most helpful in arriving at decisions regarding appropriate treatment of malignant tumors and in determining prognosis and end results [2]."
For most cancer sites, the staging recommendations were concerned only with anatomical extent of disease. Because an untreated primary cancer or tumor (T) progressively increases in size, regional lymph node (N) involvement occurs, and finally, as a result of continued extension of disease, distant metastasis (M) occurs to distant lymph nodes or organs. TNM classification and stage grouping is thus a method of designating the extent of a particular type of cancer as it is related to its natural course. For certain types of tumors, other factors, including grade, extent of differentiation, and age of the patient, were considered in staging. In the future, biological markers and other parameters may also be factors. For sarcomas, histological analysis and tumor grade may prove to be most important. In certain types of tumors, such as lymphomas, a different system for classification and staging is necessary to effectively define prognosis and to allow changes in therapy to improve outcome and quality of life.
The TNM tumor staging method has proven to be very accurate in determining prognosis; however, for GI tumors, the impact of this system on patient treatment and outcome has been held back by the limited accuracy of techniques for nonoperative staging, as well as by the limited success of various therapeutic options. Until 1 decade ago, results of chemotherapy or radiotherapy for GI tumors were disappointing because these solid tumors have low growth fractions, as well as cells in both cycling and noncycling phases. Decreased sensitivity to antineoplastic agents, which selectively kill the actively dividing cells, resulted from the marked decline in the growth fraction of GI tumors by the time they were detected [4]. Surgery for resection, palliation, or diagnosis and staging was usually attempted in all but the most advanced cases. However, as more sophisticated and effective treatments involving chemotherapy/radiotherapy combined with surgery have evolved, increased response rates and improved survival have been reported [5- 10]. Studies show that accurate staging is essential in determining the timing and dosage of these treatments for greatest efficacy. Accurate preoperative staging can now have a greater impact on patient outcome. Therapeutic procedures, even if not curative, may alter the course and life history of GI cancer.
After discussing EUS methodology, the role of EUS in planning combined chemotherapy, radiotherapy, and surgical treatment is reviewed. In addition, the uses of EUS in benign disease are addressed as they relate to establishing a diagnosis.
Equipment and Principles
Two methods of ultrasound scanning, electrical and mechanical, have been applied to intraluminal sonography. The linear sector echoendoscope electronically switches an array of piezoelectric transducers to produce a linear scan image that is parallel to the endoscope shaft. This image orientation has allowed the development of biopsy techniques. The biopsy needle can be directed to the target because it is in line with the scanning plane.
The mechanical sector echoendoscope uses a motor to rotate a single piezoelectric transducer to produce a 360 degree circular scan that is perpendicular to the endoscope shaft. Rotary electrical contacts transmit the transducer's signal to the processor. This type of scan facilitates orientation of the probe in the lumen of the GI tract. The mechanical sector echoendoscope has been the most widely used method, because rapid orientation allows faster, more efficient scanning. The Olympus (Olympus Corporation, Woodbury, NY) GF-UM systems are considered the most reliable echoendoscopes for initial scanning; however, biopsies with this method are more difficult because the needle is seen only as a single point where it crosses the ultrasound image plane that is perpendicular to the scope.
Echoendoscopes have a side or oblique angle of view, except for the echocolonoscope, which is forward viewing. The echocolonoscope has an accessory channel with optical components, which results in a wedge in the EUS image and only a 320-degree, clear sonographic image. The echoendoscope can be passed into the distal duodenum, and the cecum can be reached with the echo colonoscope. Current EUS instruments use high sound wave frequencies of 7.5 and 12 MHz, as compared with 3.5 MHz for transcutaneous ultrasonography (US) and 5 MHz for blind rectal and esophageal probes.
During EUS scanning, the transducer is guided through the GI lumen and placed directly adjacent to the area of interest. Bones, adipose tissue, and air-filled structures, which limit the sound wave imaging clarity of extracorporeal sonography, are avoided. Close proximity of the ultrasonic source to the organs imaged permits the use of high frequency, high resolution sound waves that have too short a penetration depth to be used in transcutaneous sonography. As with all sonography, the sound wave imaging plane can be oriented at any angle to bring the lesion into optimal focus, simply by turning the probe. The operator is not limited to parallel sections, usually 1 cm apart, as with computed tomography (CT).
Technique
EUS is performed in a fasting patient. Pharyngeal anesthesia and intravenous sedation are generally used but are not required. Esophagogastroduodenoscopy with a standard endoscope is performed initially because the echoendoscope has limited capabilities as an endoscope The rigid 4.2-cm tip and the oblique viewing optics of the echoendoscope make examination of the mucosa and the lumen more difficult than with standard endoscopes and similar to that with a large diameter side-viewing endoscope. Nevertheless, EUS is as well tolerated as standard endoscopy. After the initial screening endoscopy, the echoendoscope is passed under direct vision to the target area. Intraluminal gas is aspirated to increase contact with the GI wall, which maximizes available acoustic windows. The area to be imaged is brought into focus by filling a balloon surrounding the transducer at the tip of the echoendoscope with approximately 15 mL, water, thus interposing a nonattenuating acoustic coupling medium between the transducer and the surface of the GI wall. If the target area is in the wall itself, water can be placed directly into the lumen for optimal acoustic coupling. The scanning plane is then moved, using scope controls and shaft torque or push-pull maneuvers, to orient the lesion into optimal position. Techniques vary according to the indications and objectives of the examination.
Complication Rate
The complication rate in 42,105 patients surveyed [17] is low (0.05%). Two thirds of upper GI EUS complications occurred in patients with esophageal strictures. In 10 of 13 perforations, esophageal dilation had been performed immediately prior to EUS. Mortality within 30 days of EUS occurred in only 1 of 42,105 patients surveyed and was related to one such perforation. Therefore, aggressive dilation of any esophageal stricture at the time of EUS is not recommended.
Normal EUS Images
On EUS, the GI wall appears in 5 layers (Figs 1, 2). Optimal imaging of the GI wall is essential to determine the wall layers affected by disease. With the sonographic plane oriented as close as possible to perpendicular to the intestinal wall, optimal imaging and limitation of artifacts can be achieved. EUS images of organs, large vessels, and lymph nodes contiguous to the GI tract each have their own characteristic appearance.
Seven standard positions [18] are described for EUS scanning in the upper GI tract. These positions include (1) horizontal duodenum, (2) duodenum around the papilla, (3) duodenal bulb, (4) gastric antrum, (5) body of stomach, (6) fundus, and (7) distal esophagus. The normal anatomy of esophagus, stomach, pancreas, retroperitoneum, and hepatobiliary tract have been described [15,19-22]. Orientation of the images of these various organs requires an understanding of landmark anatomical structures that can be imaged from these positions (Table 1). For the esophagus, the primary landmarks include the aorta, the spine, the left atrium, the carotid arteries, the azygous vein, and the carina (Fig 3) Landmarks for the stomach include the celiac axis, the diaphragm, the liver, the spleen, the gallbladder, the left kidney, and the pancreas. For the colorectal area, the prostate, the seminal vesicles, the uterus, the vagina, the urinary bladder, the levator ani muscles, and the coxygeal bone are the primary landmarks. For the hepatic pancreatic, and biliary organs, imaging from both the stomach and the duodenum is essential to locate the bile ducts, the gallbladder, the pancreatic duct, the celiac axis vessels, the superior mesenteric artery, and the portal confluence veins (portal, splenic, and superior mesenteric). An understanding of lymph nodes associated with each organ is also crucial to understanding where to scan for spread to regional lymph nodes (N stage).
By using these anatomical landmarks for orientation, pathology in the various organs can be imaged. In patients without gastric surgery, all landmarks can be located in at least 90%. However, scanning all organs completely would take more than 1 hour. Therefore, in any given examination, certain structures or areas may not be noted if the focus is a specific anatomical region.
Figure 1
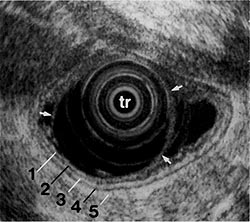
Figure 1:
EUS image showing 5 layers of the gastric wall. 1, 3, and 5 = first, third, and fifth layers are hyperechoic (white); 2 and 4 = second and fourth layers are hypoechoic (black). Transducer (tr) is surrounded by a water-filled balloon (arrows). (EUS magnification range scale = 6 cm.)
Figure 2
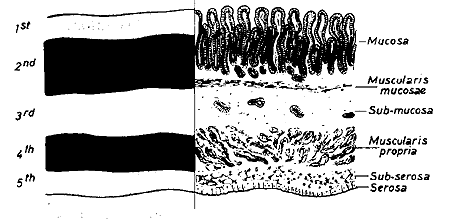
Figure 2:
Correspondence between the EUS layers and the histological layers of the normal intestinal wall. 1 st = interface between fluid in the lumen and the superficial mucosa; 2nd = lamina propria and muscularis mucosa; 3rd = submucosa and interface between submucosa and muscularis propria; 4th = muscularis propria; 5th = interface between serosa and surrounding adventitial tissue. (From [19]. Used with permission.) Table 1
Major Landmark Structures and Organs Visualized with EUS From Different Organsa
Horizontal duodenum
Aorta
Inferior vena cava
Descending duodenum
Inferior vena cava
Right kidney and renal vessels
Uncinate, pancreatic head
Superior mesenteric vein and artery
Ampulla of Vater
Common bile duct
Portal confluence
Duodenal bulb
Pancreatic head b
Bile ductb
Gallbladder
Portal veinb
Hepatic arteryb
Splenic veinb
Antrum
Inferior vena cava
Portal confluenceb
Superior mesenteric artery
Head and neck of pancreasb
Gastric body
Celiac and hepatic arteriesb
Aorta
Splenic vein and artery
Spleen
Liver
Body and tail of the pancreas
Fundus
Tail of the pancreas
Left kidney and renal vessels
Splenic artery and vein
Diaphragm
Aorta
Esophagus
Spine
Left atrium
Azygous vein
Aorta, including the arch and the aortic window
Carina
Carotid arteries
Colorectal area
Prostate, seminal vesicles
Uterus, vagina
Urinary bladder
Levator ani muscles
Coxygeal bone
*Vessels and organs seen from each upper gastrointestinal location are listed in order of appearance as the echoendoscope is withdrawn proximally from the horizontal duodenum.
bStructures usually seen well from the "withdrawn-wedged" position.
General Comments and Limitations
Table 2 summarizes the accuracy of EUS compared with CT scans for the TNM staging of various major GI neoplastic diseases. CT values are for standard dynamic CT technique with intravenous contrast agent. Data for special CT scanners and techniques such as arterial postography are not included. Accuracy is the best overall measure by which tests can be compared because it is calculated from true-positive, true-negative, falsepositive, and false-negative results. In general, gains in accuracy with EUS over other imaging methods have resulted from a significant improvement in sensitivity and, to a lesser degree, in negative and positive predictive value. There is usually only a modest improvement in specificity.
A test's level of sensitivity and specificity is directly related to what it measures. CT scans rely on measurements of size and alterations of tissue planes and organ boundaries to evaluate T stage. Thus, CT cannot differentiate T1, mucosal or submucosal, disease from T2 disease, in which the deeper muscularis propria wall layer is invaded but not breached. Accurate documentation of invasion into contiguous organs (stage T4), especially when invasion is superficial, has been equally disappointing with CT. The major contribution of EUS to staging tumors has been in the evaluation of T stage. No other imaging technique can differentiate TI and T2 lesions and document superficial invasion of contiguous vessels and organs. EUS errors in T stage do occur because EUS cannot always make the distinction between benign inflammation or fibrosis and neoplastic tissue.
Table 2
Accuracy of GI Tumor Staging and Respectability with EUS and CTa
|
EUS |
CT |
||
|
Esophagus |
|||
| T stage |
85 |
60 | |
| N stage |
80 |
55 | |
| M stage |
70 |
85 | |
|
Resectability |
80 |
55 |
|
|
Gastric |
|||
| T stage |
80 |
40 | |
| N stage |
75 |
50 | |
| M stage |
90 |
80 | |
|
Resectability |
85 |
55 |
|
|
Pancreas |
|||
|
T stage |
90 |
50 | |
| N stage |
75 |
50 | |
| M stage |
75 |
75 | |
|
Resectability |
85 |
55 |
|
|
Biliary System |
|||
|
T stage |
85 |
45 | |
| N stage |
60 |
50 | |
| M stage |
85 |
85 | |
|
|
Resectability |
80 |
60 |
|
Rectum |
|
|
|
| T stage |
85 |
70 | |
| N stage |
80 |
55 | |
|
M stage |
… |
75 |
a Values are median estimates in percent from references in text.
EUS errors in N stage can occur for similar reasons. A malignant node [23,24]can appear to have benign characteristics (Table 3) because micrometastasis may not produce any changes in the appearance of lymph nodes until disease becomes more advanced. In contrast, inflammation can cause changes in borders, echogenicity, shape, and size characteristic of a metastatic node. Overlap of criteria to differentiate neoplastic from inflammatory lymph nodes appears to be less for assessment of boundaries and echogenicity than for shape and size. CT relies primarily on size to evaluate N stage. Because a majority of metastatic lymph nodes are less than 10 mm, CT has also been insensitive to neoplastic regional lymph nodes. In contrast, because most lymph nodes larger than 10 mm are usually neoplastic, there is good specificity when CT is positive. For EUS, size resolution is not a limitation. Lymph nodes more than 3 mm are easily found [23]; however, even though EUS is superior to other imaging methods to differentiate malignant from benign inflammatory lymph nodes, criteria (see Table 3) overlap and still require improvement. Because high-frequency sound waves have a short penetration depth, a limitation of EUS is that the optimal focal range is relatively short, only 2 to 4 cm from the probe. Therefore, optimal focus of vessels or structure around a tumor larger than 5 cm may not always be achieved, and vessel or organ involvement may be missed. Also, because only portions of the liver can be visualized clearly, metastatic lesions may not be detected. However, in the part of the left lobe of the liver that can be visualized clearly, metastatic lesions less than 1 cm - missed by other imaging techniques - can be picked up by EUS [50]. EUS is very accurate in evaluation of M stage when it documents small distant malignant lymph nodes, providing that a stenotic lesion does not prevent the transducer from reaching a site such as the celiac axis. EUS can also detect small amounts of ascites not seen with other methods. An advantage of CT is that it can evaluate sites that are not accessible to EUS where tumor may spread, such as distant organs and distant malignant lymph nodes, which tend to be larger than 1 cm when associated with advanced disease. Table 3 Malignant Benign Boundaries Sharp Indistinct Echogenicity Echo-poor; Echo-rich; Shape Round Irregular Size >10mm <5mm a Criteria are useful only for frequency of 7.0 MHz or greater. Median values for M-stage accuracy of EUS and CT are difficult to estimate, since studies vary widely according to population studied (Table 2). Both EUS and CT have a high percentage of false negatives and corresponding low sensitivity; EUS because of its inability to evaluate most distant sites, and CT because of its insensitivity to small metastatic lesions. When GI tumors first present, they tend to have a low prevalence of metastases, especially if one excludes metastasis to distant lymph nodes. Prevalence of metastases is even lower in a population screened or evaluated for symptoms early. Depending on the type of tumor, 10 to 25% of patients have metastatic disease at the time of presentation, but only one-half to two-thirds of these metastases are detected by radiological imaging. Nevertheless, this low sensitivity decreases accuracy of CT or EUS by only about 10 to 15%, because prevalence of metastatic disease is low and specificity of EUS or CT is high. Thus, the relatively high accuracy values in Table 2 for M stage do not reflect the poor sensitivity of EUS or CT. In general, the sensitivity of EUS or CT in patients with positive metastatic disease is half the value of overall accuracy. Because EUS has good resolution of distant lymph node sites, but about only one third of the liver, and CT images other distant sites and the entire liver, CT and EUS are complementary for M-stage evaluation. Another limitation of EUS is that the echo pattern and features of EUS images of various diseases can appear quite similar. Consequently, differentiating criteria overlap. Some of this overlap is inherent to the ultrasound pulses; however, some of the overlap is a result of limited experience with the interpretation of EUS images. EUS will have a major role in defining abnormal areas less than 10 mm, or those mixed with fibrosis, for directed deep biopsies. Biopsy from the GI lumen through the GI tract wall can histologically establish a diagnosis less invasively than the standard surgical approach. The role of EUS in eliminating the need for surgery in certain patients clearly depends on the definition of those stages that do benefit from surgery. A lesion that has just started to invade a regional vessel or organ (advanced T stage) or a lesion with only 1 or 2 lymph nodes around and adjacent to the tumor, is certainly removable but not "truly resectable." Most esophageal tumors below the upper esophageal sphincter can be removed surgically, with the exception of those involving spread to distant organs, celiac nodes, or deep invasion of the bronchi or the aorta. Removal of a tumor that extends to the margin or into regional nodes is clearly a worse prognostic category than a "truly resectable" lesion still confined to the esophageal wall. Surgery should be attempted for tumors expected to be completely resectable. Palliative debulking of esophageal carcinoma has been shown to decrease quality of life without improving survival. Palliative surgery also delays alternative treatment [25]. However, for gastric or pancreatic carcinomas, a few positive regional lymph nodes do not appear to have as much influence on prognosis. Therefore, malignant regional lymph nodes may not be as important for surgical removal of all visible disease. T stage is probably much more important in this regard. The role of EUS in selecting appropriate patients for surgery will thus depend on alternative treatments available for amelioration of symptoms and improvement of quality of life, survival, and outcome. EUS may eventually define early tumors that are best treated initially by surgical resection, as well as those tumors that have been downstaged with alternative treatments to a resectable lesion. Because most tumors of the esophagus involve at least some part of the superficial mucosal layers, endoscopic biopsy and brush cytology are the primary methods for diagnosis. EUS will have a role in diagnosis of those unusual lesions that are only submucosal. However, histology is still required; the role of EUS is to define the area to be biopsied with special deep submucosal biopsy needles [26-29]. Although standard fiberoptic endoscopy allows direct visualization and biopsy of an esophageal lesion, it is not accurate for assessing tumor depth and extent [30]. With EUS, not only the lesion's surface, but also the size, shape, and extension of the primary tumor can be directly visualized in relation to extra-esophageal mediastinal structures and organs, as well as the presence of abnormal appearing lymph nodes. For esophageal neoplasms, the accuracy [15,30-37] of EUS in assessing T, N, and M stage is 85, 80, and 70%, respectively (see Table 2). There is a significant learning curve. In 1 study, accuracy of T-stage evaluation improved from 59% for the first 30 patients to 81% for more than 30 patients [31]. This finding is supported by the lower median accuracy of 81 % for T staging in studies reporting on less than 35 patients compared with 89% accuracy for those reporting on more than 45 patients [15]. A similar learning curve does not appear to be present for evaluation of lymph nodes, which suggests that criteria of malignant lymph nodes are easier to recognize even though these criteria (see Table 3) are unable to differentiate benign from malignant lymph nodes in approximately 20% of patients. Continuing evolution of EUS criteria for malignant lymph nodes may improve EUS accuracy. The high accuracy of EUS for local staging (T,N) has allowed major progress toward accurate preoperative TNM staging of esophageal carcinomas. Many studies have shown accuracy to have improved from less than 55% for CT to more than 80% for EUS staging. In terms of patient outcome, the most dramatic impact of EUS may be its highly accurate T staging. Data show that both prognosis and incidence of lymph node metastasis [15,32-37] correlate to the T stages, T1 to T4 (Tables 4, 5). Table 4
Criteria to Differentiate Malignant and Inflammatory Lymph Nodes
homogeneous
Nonhomogeneous
Esophagus
Malignant Diseases
Correlation of EUS T and N Stage to survival for Esophageal Carcinoma
|
Survival |
|||
|
50% |
1 year (%) |
5 year (%) |
|
|
T1 |
30 mo |
80 |
46 |
|
T2 |
13 mo |
72 |
30 |
|
T3 |
6 mo |
25 |
22 |
|
T4 |
6 mo |
17 |
7 |
|
N0 |
14 mo |
59 |
… |
|
N1 |
6 mo |
16 |
… |
a Criteria are useful only for frequency of 7.0 MHz or greater.
Table 5
Correlation of EUS T Stage with the Presence of Malignant Lymph Nodes
|
Esophagus |
Gastric |
Ampullary |
Pancreas |
Rectal |
|
|
T1 |
15 |
10 |
0 |
40 |
3 |
|
T2 |
60 |
50 |
42 |
59 |
17 |
|
T3 |
80 |
85 |
50 |
85 |
58 |
|
T4 |
100 |
100 |
100 |
… |
90 |
a Values are median estimates in percent from references in text.
Table 6
Accuracy of Local Staging for Esophageal Carcinoma: EUS Compared with CT
|
EUS (%) |
CT (%) |
|
|
T1 |
80 |
36 |
|
T2 |
78 |
36 |
|
T3 |
91 |
74 |
|
T4 |
88 |
47 |
|
N0 |
60 |
73 |
|
N1 |
87 |
48 |
|
N2 |
66 |
… |
Numerous studies have shown that neither CT nor magnetic resonance imaging (MRI) are reliable for differentiating stage I from stage II or even stage III disease. CT staging of tumor depth and invasion based on contour changes and wall thickness is frequently incorrect, especially for early stage disease [38,39]. The extent of local disease (including spread to regional lymph nodes) can only be reliably determined using EUS. In addition, compared with CT, EUS has proven to be more reliable in defining T4 tumor invasion of regional structures, such as aorta or carina, which make a tumor unresectable. Radiotherapy of a tumor that invades the trachea or bronchus is not recommended because of the possibility of development of an esophagotracheal fistula.
Studies that correlate accuracy of EUS and CT to T stage (Table 6) show that EUS is consistently more accurate; most EUS errors (73%) occur in overstaging of T2 lesions (Fig 4). Inflammatory changes can make a lesion invading the muscularis propria (T2) appear as if it just breaks through into adventitia (a T3 lesion). Data on CT scans show it is significantly more accurate for T3 lesions than for other T stages. However, because the 74% level of CT accuracy is due to higher specificity rather than sensitivity, and because EUS is more accurate even for T3 lesions, CT for T-stage disease is not clinically effective. In addition, CT scans are not particularly useful to evaluate N stage. EUS is quite sensitive in finding lymph nodes; however, because these lymph nodes may be inflammatory, an accuracy of only 60% for stage NO lesions is due to overstaging inflammatory lymph nodes as malignant. The accuracy of 70% for CT of NO lesions is due to the fact that CT misses all of these inflammatory nodes, so there can be no confusion in interpretation However, CT similarly misses many small malignant lymph nodes picked up by EUS, resulting in an accuracy of only 48% for CT scan as compared with 87% for EUS in evaluating N1 and N2 disease.
Figure 4
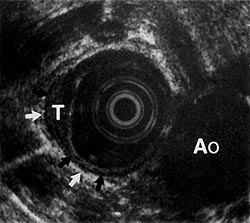
Figure 4:
Hypoechoic tumor (7) (measuring 6 mm deep, 19 mm in circumference) extending into the muscularis propria layer (black arrows). The adventitial layer (white arrows) is intact. Ao = aorta. (EUS magnification range scale = 6 cm.)
One major limitation of EUS in staging esophageal neoplasms is the larger diameter of the echoendoscope, which is unable to pass through at least 25% of strictures, even though they may be traversed with thinner endoscopes.
Several prototype echoprobes have been designed to attempt to deal with this problem. One type of probe can be inserted through a 3.5-mm biopsy channel of a standard endoscope. Both the accuracy and the utility of this probe is limited by the very small acoustic field of view and the limited penetration of its 20-MHz sound wave frequency [40].
Another approach is based on a blind echoprobe that can be passed over a guide wire through esophageal strictures [41 ]. The diameter required to pass this instrument is less than 9 mm (33 French), compared with the usual 12.6-mm (40 French) diameter of the echoendoscope. This probe may prove useful in patients with apparent advanced disease that cannot be documented by other nonoperative techniques. Its ability to detect celiac nodes not seen on CT would have a significant impact on selection of radiotherapy, endoscopic palliation, or chemotherapy over surgical therapy.
In terms of clinical outcome, however, inability to pass the echoendoscope through a stricture may not be significant [15,32,42,43] because (1) stenotic lesions are generally advanced (90% invade through the muscularis propria [T3 or T4] and have spread to many lymph nodes or distant sites); (2) EUS often finds malignant lymph nodes proximal to the stenosis; (3) CT scans will find metastasis to distant sites, which occurs more often in lesions with an advanced T stage (EUS up to the stenosis plus CT scan will provide as accurate an evaluation as EUS past the stricture in more than 95% of patients); and (4) as described, aggressive dilation of these strictures at the same session as EUS is associated with a high risk of perforation and is not recommended [42]. Two studies looked at selective passage of stenotic esophageal tumors [43,44] with and without dilation. No complications were reported. In 1 study [43], EUS was very accurate, both for lesions with stenosis easy to pass through, and for those that could not be traversed (Table 7). EUS was least accurate in staging lesions with stenosis that was "difficult to pass," but EUS accuracy was still better than CT for these difficult-to-pass lesions. In another study, lesions through which a 9-mm endoscope was unable to pass (16%), were not dilated; all patients had T4 disease. In 62% of patients, the echoendoscope passed beyond the stricture without dilation, 21% of patients had tumors that were dilated to allow the echoendoscope to pass. In this latter group, only 2 patients (15%) did not have a T3 or T4 lesion, and 40% had malignant celiac nodes. From this study it appears that dilation can be performed if needed over several sessions in the usual manner without significant complications.
Table 7
Influence of Tumor Stenosis on the Accuracy of EUS Compared with CT in the Preoperative T Staging Of Esophageal Cancer [43].
|
Passage with Echoendoscope |
||||
|
Good |
Difficult |
Impossible |
Total |
|
|
EUS |
12 (92) |
6 (46) |
13 (87) |
31 (76) |
|
CT |
9 (69) |
5 (38) |
6 (40) |
20 (49) |
EUS accuracy is high in defining resectable lesions confined to the esophageal wall (TI, T2) without spread to other sites (see Fig 4). In this regard, EUS is better for staging adenocarcinomas (89-92%) than squamous carcinomas (64-84%) because of the tendency of squamous lesions to have submucosal microscopic lymphangiosis carcinomatosa beyond the tumor margin, detectable only on histological analysis [35,36]. Early tumor stages are generally the best candidates for surgical resection; however, for those carcinomas confined to the mucosa, alternative local treatments, such as strip biopsy, photodynamic therapy, or endoscopic dissection, are alternatives in which EUS may help in directing the most appropriate treatment.
Currently, the combination of CT plus EUS [45] provides very accurate preoperative staging (85% compared with 60% for either method alone for overall stage accuracy). Whether all patients with small lesions will need a CT scan or whether all patients with extensive large lesions need EUS will be determined in prospective studies that assess cost-effectiveness.
Multimodal selective treatment concepts can now be applied on a rational basis. EUS will most accurately identify patients with unresectable tumors who are candidates for preoperative chemotherapy and radiotherapy. Only those patients responding to treatment become candidates for surgery, assuming the patient is not functionally inoperable. Hilgenberg and colleagues [6] reported the best results: 36% 5-year survival when chemotherapy, radiotherapy, and surgery were combined.
The impact on overall patient outcome will clearly improve as selective treatment improves prognosis and quality of life.
For detection of anastomotic recurrences of esophageal carcinomas, EUS has been shown to be sensitive but somewhat nonspecific [45,46]. It is most valuable in those patients in whom recurrence is suspected based on other standard techniques, but in whom endoscopic biopsy is negative, which occurs in approximately 25% of recurrences. For diagnosis of local recurrence of esophageal carcinoma, EUS has a sensitivity of 95%, a specificity of 80%, a positive predictive value of 88%, and a negative predictive value of 92%.
EUS evaluation of lymph nodes due to metastasis from lung carcinomas appears to be useful [47]. Excluding right superior mediastinal lymph nodes, the sensitivity and specificity were 81 and 98%, respectively, in detecting malignancy. When anthracosilicosis is present, results are not nearly as good, and directed biopsy is required to confirm diagnosis.
EUS is also useful for monitoring the response to chemotherapy and radiotherapy. Although it can be difficult to differentiate residual malignancy from fibrosis by EUS, estimation of tumor size, and therefore therapeutic response, can be made. However, the most appropriate use of EUS for patients undergoing treatment needs further evaluation. Radiotherapy induces inflammatory and fibrotic changes that complicate interpretation of EUS [48]. EUS will be uncertain or incorrect in 1 of 3 patients even after waiting 3 months after radiotherapy [33].
Benign Diseases
EUS has been used in the evaluation of various motor disorders [49], including scleroderma and achalasia. Various abnormalities of the esophageal wall, including thickening or replacement of various layers, have been described. These findings, however, are found inconsistently because artifacts can easily result when scanning the area around the lower esophageal sphincter. Oblique scanning, balloon compression, and rapid passage of the scope through the abnormal area can make accurate evaluation of the thickness of each layer in the GI wall difficult. However, when malignancy causes a disturbance of the 5-layer wall architecture in asymmetrical patterns, malignancy can usually be distinguished from primary achalasia. In 1 study [49], 6 of 36 patients with manometric signs of primary achalasia were found on EUS to have either malignant or benign tumors. In patients found to have benign submucosal tumors of the esophagus, manometric findings consistent with various motility disorders can be found in more than 50%. EUS therefore appears to be most valuable in finding abnormalities that may mimic primary motility disorders. Tumors causing pseudoachalasia can easily be detected with EUS. It should therefore be performed in patients in whom initial evaluations are not clearly diagnostic and there is suspicion that a tumor may exist.
The inability of EUS to reliably differentiate inflammatory from malignant tissue causes problems in the evaluation of a smooth esophageal stenosis. There has been no clear demonstrated value of EUS in evaluating peptic esophageal strictures, Barrett's esophagus, or other reflux-related diseases [15]. EUS may have a role in directing repeat biopsy of suspicious areas when initial biopsies are negative, but, because of the low accuracy in differentiating inflammatory or fibrous tissue from malignant tissue, EUS will have a limited role in these diseases. Once a diagnosis of malignancy is made, however, staging will be as accurate as with other malignancies of the esophagus.
Gastric
Malignant Diseases
Screening endoscopy with biopsy is again regarded as the procedure of choice to evaluate lesions suspicious for carcinoma of the stomach. Except for tumors that do not break through the mucosal surface (e.g., linitis plastica), conventional endoscopy is superior to EUS for diagnosis. EUS has been shown to be more accurate than any nonoperative technique to stage gastric neoplasms [1,15,50]. The accuracy of local staging of gastric neoplasms with EUS is slightly less than for esophageal neoplasms (see Table 2). CT scan is important in evaluating the M stage of disease; however, endoscopic ultrasound is up to 6 times more accurate in staging tumors than CT scan, if one takes into account early lesions that CT scan cannot accurately stage. The thickness of the gastric wall can be affected by other pathology, most commonly acute and chronic inflammation. In addition, small regional lymph nodes are frequently missed by CT. CT scan is useful in evaluating progression of the tumor beyond regional lymph nodes, such as in the celiac, gastroduodenal, and gastrohepatic sites. When tumors are large enough to distort the gastric anatomy, it may be more difficult to orient the EUS probe to accurately evaluate tumor stage.
For gastric tumors, the clinical utility of EUS is directly related to available stage-dependent treatment. If stage-dependent protocols are applied, EUS staging is mandatory. EUS has been shown to be 85% accurate in predicting resectability. EUS decreases the need for exploratory laparotomy for patients with gastric carcinomas [51].
Prognosis depends on depth of tumor invasion, lymph node metastasis, and distant metastasis. Initial EUS T stage has been shown to correlate with spread to lymph nodes [51], survival, and frequency of recurrence [52] after surgical removal of the lesion (see Table 5; Table 8). In a group of patients who on preoperative staging had nonresectable gastric tumors, 50% were found to be resectable at laparotomy following preoperative chemotherapy. Median survival of these unresectable gastric carcinomas was 24 months with treatment. In another multicenter study comparing postoperative chemotherapy with no treatment, patients with advanced T-stage tumors had a significant increase in 5-year survival with chemotherapy, whereas those with early TI or T2 stage tumors did not benefit [25]. It is unknown whether patients with T2 lesions (Fig 5) will benefit more from radical versus limited resection or how these two different surgical approaches would affect quality of life.
Accuracy for each T and N stage is quite high for EUS: 80% for T 1, 65% for T2, 87% for T3, 79% for T4, 84% for NO, 74% for N 1, and 63% for N2. EUS can detect metastatic spread to distant (N2) lymph nodes [51]. There are, however, certain limitations of EUS staging for gastric carcinomas. Lesions that infiltrate only into the subserosa can be overstaged because associated fibrosis and inflammatory changes or ulceration can obscure the border between the subserosa and the serosa; differentiation between T2 and T3 lesions is therefore difficult, resulting in only a 55% accuracy for T2 lesions [15,36,53]. Parts of the proximal stomach not covered by serosa add to EUS image interpretation difficulties. Ulceration occurring in early carcinoma complicates differentiating malignancy from fibrosis and inflammation with EUS; therefore, carcinomas involving only the mucosa may at times not be distinguished from carcinomas involving the submucosa. This differentiation is important in selecting patients who may be amenable to endoscopic treatment [54,55].
Table 8
Correlation of EUS T Stage with Survival Recurrence of Resected Gastric Carincoma a
|
50% Survival (mo) |
Recurrence (%) |
|
|
T1 |
41 |
0 |
|
T2 |
29 |
20 |
|
T3 |
20 |
73 |
|
T4 |
7 |
100 |
Data from [52].
a Median follow-up time of 25 months.
Figure 5
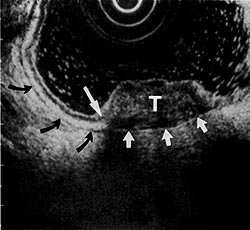
Figure 5:
Mixed hypoechoic gastric tumor (T) disrupting the normal 5-layer architecture at this point (long arrow). Tumor extends into the hypoechoic muscularis propria layer (black arrows). The hyperechoic serosal layer (short arrows) is intact. (EUS magnification range scale = 6 cm.)
Lymphoma
The clinical and morphological features of gastric lymphomas can resemble those of carcinomas. Because lymphomas tend to be submucosal and to ulcerate as disease progresses, abnormalities of the stomach can be detected in up to 75% of patients. However, the diagnosis is made with contrast radiography in less than 20% of patients. In addition, endoscopic biopsies correctly diagnose lymphoma in only 40 to 60% of patients. Using biopsy techniques that obtain submucosal tissue [26,281 in conjunction with EUS [56-60], preoperative diagnosis should be possible in almost all patients. Because of the high accuracy of deep biopsy technique plus EUS staging, laparotomy should no longer be used to stage gastric lymphomas. The accuracy of EUS for depth of invasion of gastric lymphomas evaluated by EUS has been reported to be 96% for T 1, 67% for T2, 100% for T3, and 80% for T4. For N-stage evaluation, EUS sensitivity for perigastric lymph nodes was 44%, specificity was 100%, and positive and negative predictive values were 100 and 72%, respectively [57].EUS can be used to monitor response during chemotherapy and radiotherapy [31]. Only EUS is able to demonstrate complete disappearance of intramural infiltration and reappearance of the normal 5-layer wall architecture. However, there may still be microinvasion in the submucosa that does not cause alteration of the architecture. Deep biopsy would be the only nonoperative method of determining this microinvasion. Recent studies [57] have shown that frequent repeat endoscopy with deep biopsy is important in patients in whom lymphomas are suspected but have not been diagnosable by biopsies or abnormal EUS findings. These patients, in whom endoscopic abnormalities are minimal, probably represent less than 5% of those with gastric lymphomas. In a study [59] in which patients with infiltrative gastric lymphomas were evaluated with sequential endoscopies over several months, only 33% were diagnosed on the first endoscopy with biopsy, but follow-up endoscopy plus biopsy at 4-week intervals diagnosed all but 1 patient without surgery. EUS has been shown to make a significant impact on the rate of total gastrectomy, decreasing the rate from 65 to 10% by accurately defining the area of involvement by lymphoma [56]. Because of the high accuracy of preoperative staging currently available with EUS for gastric lymphomas, selective treatment according to stage becomes a realistic option.
Prior to EUS, accurate staging was possible only through surgery, because CT scans were negative except for far advanced disease. Currently, there is no standardized, accepted therapy for primary gastric lymphomas. Although surgical resection of the lesion was an essential part of therapy in most studies, treatment of high-grade malignant lymphomas with only chemotherapy has achieved similar survival in a study reported by Salles and associates [61]. Gobbi and colleagues [62] also found no difference in survival rates between patients undergoing primary resection versus those not undergoing surgery. Considering the significant long-term morbidity and mortality associated with surgery for gastric lymphomas, the high rate of extraabdominal relapses when chemotherapy and radiotherapy are not included as part of the treatment, and, finally, the improved quality of life when the stomach is not resected, chemotherapy alone will certainly find a place in treatment of specific stages of lymphomas. Recently, a large multicentered trial showed that the treatment of choice is CHOP (cyclophosphamide/doxorubicin/vincristine/prednisone) compared with other chemotherapy regimens [63].
Benign Disease
There has been no clear role established for the use of EUS in evaluating gastric polyps. EUS cannot differentiate malignant from inflammatory epithelial polyps unless there is infiltration into layers beyond the submucosa [641]. In polyps, EUS can define large vessels that could pose a risk for endoscopic polypectomy [65]. EUS evaluation of suspicious gastric ulcers with a biopsy negative for malignancy has not proven to be useful in making any further diagnosis. Changes of the EUS wall layer resembling malignancy regularly occur in the course of ulcer disease, making EUS differentiation between benign and malignant wall changes very difficult, if not impossible [66]. A few reports [67] indicate that the EUS pattern of benign gastric ulcers may help to predict healing rates and therefore identify ulcers that would tend to be refractory to standard treatment.
EUS appears to have a major role only in patients with giant gastric folds. EUS can define areas of abnormality that are most likely to yield a positive deep biopsy. Gastric wall thickening involving only the first 2 mucosal layers is characteristic of hyperplastic wall thickening in Ménétrier's disease. Mucosal wall thickening can also occur in early lymphomas, but lymphomas generally cause a thickening of the submucosal layer.
One important use of EUS in evaluating large gastric folds is to exclude gastric varices. The portal venous system can be well visualized with EUS [68]. Endoscopy has been shown to be superior to EUS for grading esophageal varices; however, EUS has been shown to be far superior to endoscopy or other imaging techniques in detecting gastric varices. EUS has been shown to diagnose gastric varices in up to 80% of patients when gastric varices were not diagnosed by standard endoscopy. However, other than the use of EUS to diagnose a gastric varix when a submucosal lesion is seen, there are no established clinical indications for the use of EUS in portal hypertension.
Colorectal
Malignant Disease
The combination of EUS (or transrectal ultrasound) plus CT is the most practical and accurate approach to staging rectal carcinoma. EUS has been shown to be highly accurate in determining the TNM stage of colorectal carcinomas; it is far superior to CT scan alone. CT scans, however, are essential to evaluate for metastatic disease to distant organs. EUS accuracy of local staging for rectal carcinomas is 94% for T 1, 73% for T2, 92% for T3, 94% for T4, 79% for NO, and 74% for N I disease.
EUS has contributed significantly to the selection of different preoperative protocols for patients with colorectal tumors. Application of various therapeutic protocols that depend on the stage of the lesion is now feasible. For low rectal lesions that can be palpated, staging accuracy with digital evaluation is approximately 70 to 80%. A digital rectal examination therefore sets a very high level of accuracy for distal lesions that has proven difficult to improve on, even with extensive technological evaluation [69,70].
Although EUS has resulted in improved accuracy of evaluation of N stage, diagnostic problems still exist be cause more than two thirds of malignant lymph nodes are smaller than 5 mm, causing a low sensitivity [71]. Data from Rifkin and co-workers [72] comparing EUS and CT showed sensitivity of'45 versus 27%, specificity of 93 versus 88%, positive predictive values of 71 versus 46%, and negative predictive values of 82 versus 76%, respectively. Their data show the problem of detection of malignant lymph nodes. EUS, which is almost twice as sensitive as CT, still only picks up 501/1c of malignant nodes.
Most patients with carcinomas of the colon will undergo attempted resection unless there is evidence of metastatic disease. With introduction of new surgical techniques, if the lesion is localized without spread to lymph nodes, the operation performed will depend on accessibility and size of the tumor. An 89% 5-year survival has been reported for local removal of early lesions. This value compares with a 5-year survival of only 73% for abdominal perineal resection, and 76% for an anterior resection of similar T1, T2, and NO lesions [70].
Availability of neoadjuvant irradiation and expertise in local surgical procedures will be the determining factors that shift treatment from radical resection in all patients to other modalities according to staging. Carcinomas could be resected laparoscopically; local excision and sphincter-saving procedures may be attempted for rectal tumors. Patients who have been shown by EUS to initially have unresectable rectal carcinoma have been treated with preoperative radiotherapy; 90% have subsequently become resectable [10]. Accurate staging will also be important for lesions in the middle and lower third of the rectum. An increasing number of sphincter-preserving operations can now be performed for amenable lesions (i.e., those that do not invade the sphincter complex, pelvis, or adjacent organs).
A final use of EUS in colorectal tumors is for evaluation of an anastomosis at a prior resection site when there is suspicion of recurrence but biopsies are negative. EUS is essential in these patients because CT cannot reliably differentiate postoperative changes from tumor recurrence until the disease is well advanced. Local recurrence occurs in 20 to 30% of resected colorectal tumors. In only approximately one third of these tumors does recurrence involve the mucosa, which could be diagnosed with standard mucosal biopsy. EUS has been shown to accurately predict colorectal tumor recurrence [73], similar to prediction of anastomotic recurrences for resected esophagogastric lesions.
Benign Disease
Because endoscopic ultrasonography relies on alterations of the 5-layer architecture to diagnose abnormalities, it can precisely localize lesions such as adenomas, ulcers, submucosal tumors, and anal sphincter defects and determine whether any layers are destroyed [22,73-75]. However, EUS cannot reliably distinguish between a benign adenoma on the surface of the mucosa and a localized carcinoma that has not disrupted deeper layers. Once the integrity of deeper layers becomes disrupted, EUS becomes quite accurate in staging a lesion However, inflammatory changes due to benign ulcerations can mimic disruption of integrity of deeper layers and may result in an incorrect diagnosis or stage. Biopsy confirmation of the histological nature of the lesion is there fore essential to accurately assess colon wall abnormalities
Thickening of all layers of the colorectal wall or absence of superficial layers can be seen in ulcerative colitis [74]. Benign pericolic lymph nodes can also be detected by EUS. Therefore, it is unlikely that EUS will have a role in finding areas suspicious for malignancy in patient with ulcerative colitis.
EUS has made an impact in the management of patients with Crohn's disease by improving detection and mapping of abscesses and fistulas [22,74,75]. These lesions can be found even in patients without symptoms suggestive of anal-rectal disease. For detection of pararectal abscesses, sensitivity of transrectal ultrasound appears to be more than 90%, compared with less than 60% for CT. EUS has also been reported to differentiate transmural inflammation from mucosal inflammation; however, the utility of this information has yet to be determined.
Submucosal Tumors of the GI Tract
EUS is the only imaging technique that can delineate the histological layers of the GI tract wall. Therefore, evaluation of submucosal tumors becomes one of the main indications for EUS because it can accurately define the origin, extent, and depth of invasion of submucosal tumors. CT does not further characterize these lesions unless they are quite large.
Although submucosal tumors of the GI tract are most often found on barium radiological examination or endoscopy, standard endoscopic biopsies usually do not demonstrate any abnormality because the lesion is under the mucosa. In addition, it is often impossible to determine whether a mass below the mucosal surface is an extrinsic or intrinsic tumor, a vascular structure, or compression from an adjacent organ. EUS can make this differentiation very accurately (98%), compared with barium radiographs (75%), endoscopy with biopsy (67%), or CT (53%) [15]. EUS will detect a submucosal tumor in nearly 100% of patients as compared with 92% for barium radiographs and 67% for CT. Failure to demonstrate a submucosal tumor with EUS in up to 5% of patients occurs mainly for technical reasons; placement of the transducer on the tumor may be difficult when the location of the tumor is on the lesser curvature or in the cardia.
If a tumor is found, an EUS-directed submucosal deep-needle biopsy or aspiration can be performed to make a histological diagnosis [26-291]. This type of biopsy is essential because sonographic features to differentiate benign from malignant tumors overlap. Tumor destruction of the wall architecture appears to be the most specific sign of malignancy, particularly for primary or metastatic submucosal carcinomas. Biopsy with EUS distinguishes these types of tumor from mesenchymal tumors [28].
Mesenchymal tumors less than 3 cm are almost never malignant; those larger than 4 cm have a higher probability of malignancy [76]. When the lesion is in the fourth EUS layer (i.e., the muscularis propria), differentiation between a leiomyoma, a leiomyoblastoma, and a leiomyosarcoma is very difficult without the presence of a disrupted wall architecture or abnormal lymph nodes. Some leiomyomas arise from the second deep mucosal EUS layer. Defining this superficial location with EUS (Fig 6) may allow endoscopic removal of certain mesenchymal tumors with new endoscopic tools.
p align=center>Figure 6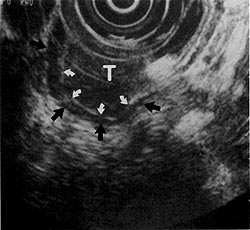
Figure 6:
Hypoechoic submucosal tumor (7) above the muscularis propria (black arrows) layer. A submucosal hyperechoic margin is noted (white arrows) between the tumor and the muscularis propria layer. (EUS magnification range scale = 6 cm.)
Hypoechoic mesenchymal tumors can be differentiated from lipomas and fibromas, which are usually echo-rich, well demarcated, and located within the submucosal third EUS layer. Another lesion in the submucosa
that can be clearly delineated by EUS is an aberrant pancreas. Cysts in the submucosa have a well-demarcated, echo-free appearance. Carcinoid and lymphoma are also found in the submucosa and can occasionally appear as localized lesions. Varices in the gastric wall have an echo-free appearance; continuity with varices external to the gastric wall establishes the diagnosis.
Organs that have been shown to cause compression of the gastric wall, simulating a submucosal tumor, include spleen, liver, kidney, gallbladder, and vessels around the celiac axis. In addition, tumors of the pancreas, enlarged abnormal lymph nodes due to tumor or inflammation, tumors of the liver, and retroperitoneal tumors can cause an impression on the gastric wall resembling a submucosal tumor. In the esophagus, the bronchus, the thoracic spine, and the aorta can simulate a submucosal tumor. These "tumors" can all be diagnosed with EUS.
Surgical removal of true submucosal tumors may be indicated by suspicious biopsies, location, endoscopic ultrasound findings, or symptoms that suggest malignancy. When benign lesions are small (< 3 cm), observation with EUS is a reasonable approach, especially if negative histology has been obtained. When lesions are smaller than 2 cm and located in the esophagus, biopsies may not be necessary, and lesions can be followed with endoscopic ultrasonography alone, because yield tends to be lower and complication rates higher for biopsies of these small esophageal lesions [28]. Histological confirmation of rectal submucosal lesions is mandatory because more than 50% are malignant [22].
Pancreas and Biliary Tract
Malignant Disease
Because of the difficulty in obtaining accurate imaging of the pancreas with other methods, EUS may have its greatest impact in the evaluation of this organ [77,78]. Studies show that EUS is more accurate than any other test, including ultrasound, CT, MRI, ERCP, and angiography, for detection and staging of pancreatic tumors. EUS is also more accurate than CT or other tests for predicting resectability of a pancreatic mass. The high resolution images of EUS make it possible to visualize very small lesions, ductal abnormalities, and calcifications not seen with other methods. The accuracy of T staging is consistently good for both pancreatic and ampullary neoplasms (Table 9). T stage also correlates to presence of lymph nodes (see Table 5) and survival (Table 10).
The high accuracy of real-time EUS of the pancreas is primarily due to both its unsurpassed resolution of the parenchyma of the pancreas and its capability of evaluating and integrating (during the same examination)mucosal, vascular, ductal, and parenchymal abnormalities caused by disease. To obtain information about these 4 types of abnormalities, 4 separate tests would otherwise be required: (1) endoscopy for mucosa, (2) venogram or arteriogram for veins and arteries, (3) ERCP for pancreatic and bile ducts, and (4) CT or standard sonography for the parenchyma and the surrounding lymph nodes. Indications for EUS of the pancreas now include (1) staging potentially resectable tumors, (2) detection of endocrine tumors, and (3) evaluation of equivocal abnormalities in the pancreas present on other studies.
Table 9
EUS Accuracy of T Staging for Pancreatic Carcinoma
|
Ampullary (%) |
Pancreas (%) |
|
|
T1 |
67 |
100 |
|
T2 |
92 |
88 |
|
T3 |
87 |
93 |
|
T4 |
100 |
… |
Table 10
Correlation of T and N Stage to Survival For Pancreatic Carcinoma
|
Survival |
||
|
50% |
1 year (%) |
|
|
T1 |
24 mo |
76 |
|
T2 |
9 mo |
48 |
|
T3 |
8 mo |
25 |
|
T4 |
8 mo |
18 |
|
N0 |
11 mo |
… |
|
N1 |
6 mo |
… |
EUS has clearly emerged as the most accurate test for imaging pancreatic disease. Techniques to image the pancreas have been described [20]. If the entire pancreas and other retroperitoneal structures are to be visualized, the echoendoscope must be inserted to the horizontal duodenum. Frequently, to achieve optimal scanning of the pancreas and the surrounding vessels, the transducer must be anchored in the duodenal bulb by inflating the balloon surrounding the transducer with water. Then, by withdrawing the scope, the pylorus will be pulled back and the lesser curve of the stomach shortened. This "withdrawn wedged" position improves control of the angle of scanning of the pancreas [20] (Figs 7,8,9).
Conventional initial evaluation of a patient with a suspected pancreatic carcinoma usually consists of transcutaneous US, CT scan, or both. A direct relationship exists between mass size and the sensitivity and specificity of these tests for detection, staging, and assessment of resectability. For patients with small lesions, for whom resection is generally considered to be the best palliation and may offer the best chance of improved survival, preoperative evaluation is most difficult.
EUS can identify localized pancreatic, biliary, or peri- ampullary neoplasm and differentiate it from disease with regional lymph nodes, metastatic spread, or, most importantly, extrapancreatic extension into blood vessels [79,80]. The retrospective study by Yasuda and col leagues [81] reports on the detection rate of pancreatic tumors by EUS, CT scans, standard US, angiography, and ERCP. An important aspect of the study concerns the size of the tumor. For lesions that were larger than 3 cm, all tests were quite good at detecting tumors: EUS detected 100%; the other tests detected 82 to 97%. How ever, for tumors that were less than 3 cm, the difference in detection rate between EUS and other tests increases dramatically: 100% compared with 24, 57, 29, and 14%, respectively.
Figure 7
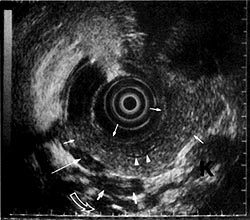
Figure 7:
EUS image of the normal pancreas from the body of the stomach. The pancreas has a finely granular echoic pattern that is characteristically very homogeneous.
Short thin arrows= body of pancreas;
arrowheads = part of the normal diameter (1 mm) pancreatic duct in the tail;
curved open arrow= renal vein;
short closed arrows = splenic vein;
long thin arrow= splenic artery.
K = upper pole of the kidney. (EUS magnification range scale = 9 cm.)
(From [84]. Used with permission.)
Figure 8
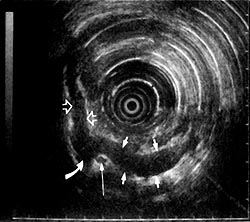
Figure 8:
EUS image from the duodenal bulb with the transducer in the "withdrawn-wedged" position. Major arteries and portal confluence vessels around the pancreas are seen. Short open arrows = portal vein; curved closed arrow = superior mesenteric vein; short closed arrows = splenic vein; long thin arrow = superior mesenteric artery. (EUS magnification range scale = 12 cm.)
(From [84]. Used with permission.)
Figure 9
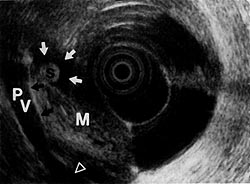
Figure 9:
Mixed echoic mass (M) disrupting the interface (black arrows) of the portal vein (Pv), indicating invasion(open arrow). A small common bile duct stone (S) is noted in the hypoechoic dilated common bile duct (white arrows). (EUS magnification range scale = 9 cm.)
Since publication of Yasuda's and colleagues' [81] ret rospective study, many prospective studies comparing EUS to other imaging tests have shown that EUS is more accurate than any other test for diagnosis, staging, and predicting resectability of pancreatic tumors [78]. Most studies excluded patients with suspected bile duct stones and evaluated only patients suspected of having pancreatic disease. CT scan and standard US have a low sensitivity in finding bile duct stones. Including patients with bile duct stones into a study will naturally favor EUS because EUS has been shown to be very accurate in finding common bile duct stones [82]. Occasionally, the presentation of bile duct stones can mimic a tumor, but more than 80% can be detected using clinical and laboratory criteria alone [83]. Nevertheless, the greater accuracy of EUS to evaluate tumors, as compared with other imaging techniques, was also found in studies that did not include patients with common bile duct stones.
There are only 2 reports that studied the issue of clinical utility by prospectively comparing findings of EUS to the combined findings of CT plus ERCP to differentiate malignant from inflammatory pancreatobiliary tumors. One study [84] showed that EUS was superior, and the other study [85] showed that CT plus ERCP was better. In the study by Rosch and associates [85], mean tumor size was 4.5 cm (range, 2-20 cm). This study pointed out that, as reported by Yasuda and colleagues [81], EUS was superior to CT or ERCP for tumors measuring less than 3 cm. In the study by Snady and co-workers [84], in which EUS was superior to CT plus ERCP to differentiate malignant from inflammatory tumors, mean tumor size was less than 3 cm (maximum, 5 cm). Therefore, the primary difference between the 2 studies was the size of the lesion. Thus, in combining the data from both studies, it appears that the larger a tumor is, the less the difference between CT plus ERCP compared with EUS will be. For smaller, potentially resectable, tumors, the greater the difference will be in favor of EUS. Correctly identifying patients with resectable pancreatic carcinomas and no metastatic disease or vessel involvement would spare many others a major operation.
The size of a pancreatic tumor is the primary factor in determining an algorithm for diagnosis and treatment (Fig 10). Initial evaluation of suspected pancreatic carcinomas with ultrasound or CT scan will accurately stage the extent of disease for lesions larger than 5 cm. In pancreatobiliary disease, the accuracy of CT scan relies primarily on distortions of contiguous fat planes in the area. Difficulties with CT arise in evaluating small tumors. Fat planes may be unaltered because the tumor is still confined to the pancreas, or because there is an associated accompanying loss of fat planes with tumor-associated weight loss. Also, the density of small tumors is often so similar to that of the normal pancreatic parenchyma that it remains undetected despite thin-section CT scan. Although CT scan can visualize the pancreas in more than 90% of patients, a tumor must be at least 2 to 3 cm for probable detection. A mass may not even be detectable by CT scan in approximately 20% of patients preoperatively considered to have resectable carcinoma before EUS assessment.
EUS is unlikely to provide additional information for patients with large tumors, because the EUS probe cannot always be brought close enough to the lesion to bring its margins into optimum focus. Diagnosis with fine-needle biopsy followed by palliative bypass of the biliary obstruction with endoprosthesis or surgical bypass is straightforward. These patients can then be treated with combined modality therapy.
For tumors that appear localized and are less than 5 cm on standard US or CT scan, EUS will provide the information required to select the most appropriate management plan. Performing EUS early in the evaluation of such patients is likely to be effective in terms of clinical utility. Reviewing the data from various studies shows that endoscopic US will significantly change the management in approximately one third of patients when used in the appropriate clinical setting. If EUS shows the tumor is resectable, then laparoscopy to exclude small liver metastases, generally not seen on CT, may prove to be beneficial prior to attempted resection.
EUS detects vascular involvement by pancreatic tumors as accurately as angiography and is unsurpassed in accuracy of determining resectability [80,86,87]. Because of the high accuracy of EUS in predicting resectability, EUS will be important in directing patients to alternative treatment to surgery. Patients with pancreatic carcinomas that are unresectable due to local invasion of blood vessels (T3) have been shown to respond to combined chemotherapy and radiotherapy. Median survival with combined chemotherapy and radiotherapy matches survival of a contemporary group of less advanced TI tumors resected without combined chemotherapy and radiotherapy [9]. For patients with T3 tumors responding to combined chemotherapy and radiotherapy, in whom surgery is being considered because of CT-demonstrated downstaging, EUS found an additional 40% still unresectable [8]. EUS can assist in evaluating response to combined chemotherapy and radiotherapy and predict resection status of pancreatic tumors. As shown in the algorithm, EUS may also have a role in monitoring patients treated with chemotherapy and radiotherapy.
EUS can detect and stage small pancreatic tumors of all types, including endocrine tumors, even those not localized by other tests [88]. EUS accuracy in detection of endocrine tumors is more than 85%, as compared with less than 50% for angiography, CT, or ultrasound [88-90]. Without EUS, endocrine tumor localization will fail in more than 50% of patients, even when adding MRI, angiography, and venous sampling. Intraoperative US is more complex and is unlikely to show any significant advantage over preoperative EUS, although no comparative study has yet been reported. Because of its accuracy, convenience, and low complication rate, EUS appears likely to replace all other tests as the initial method to evaluate patients diagnosed on clinical signs and laboratory tests as having an endocrine tumor. Localizing duodenal wall gastrinomas is a limitation of EUS. Intraoperative transillumination of the duodenum has been shown to be useful for detection of extrapancreatic endocrine tumors [91].
As with primary pancreatic tumors, EUS has been demonstrated to be highly accurate in staging gallbladder, bile duct, and ampullary tumors [1, 15,92-96]. Until an ampullary malignancy causes changes in the underlining wall layers, EUS cannot differentiate early carcinomas from adenomas. EUS, however, will identify such a lesion as a cause of duct dilation or related symptoms. Inflammatory changes can cause problems in diagnosis and staging, similar to problems with diagnosing and staging lesions in other parts of the GI tract. Biliary tract tumors present a problem for N-stage evaluation with EUS because most lesions, even in the early stage, have associated inflammatory nodes. This factor results in a high false-positive rate for malignant lymph node identification. T-stage accuracy for EUS is 85% for ampullary and biliary tumors and 80% for gallbladder neoplasms. EUS N-stage accuracy is 75% for ampullary tumors and 90% for gallbladder tumors but only 55% for biliary tumors. However, for US and CT, staging accuracy is far less, because these tests merely detect the tumor in 20 to 50% of patients and do not further characterize the lesion.
Small probes can be used to obtain intraductal images. Their clinical utility has not been demonstrated [21, 97,98]. As these probes improve, their role in evaluation of the biliary duct system should expand. For the pancreatic duct system, these probes will probably not be clinically useful.
Benign Diseases
On EUS, the normal pancreas has a homogeneous, fine, granular, echogenic pattern and a smooth border (see Fig 7). The pancreatic duct is smooth and less than 2 mm in diameter. The ventral pancreas tends to be less echogenic than the dorsal pancreas in many patients. Compared with EUS, a successful ERCP will provide superior imaging of the common bile duct and pancreatic duct. However, even when ERCP does not visualize a part of the pancreatic duct or common bile duct, this area will usually be seen well on EUS.
EUS can detect chronic pancreatitis in patients in whom other tests have not shown diagnostic findings. EUS findings in chronic pancreatitis have been reported [97,99]. These studies describe many duct and parenchymal findings such as increased duct size, duct irregularity, increased duct wall echogenicity, side branch visualization or dilation, strictures with dilation of the main pancreatic duct, intraluminal echoes due to calculi and protein plugs, and disruption of the duct with cyst formation. Parenchymal changes include enlargement of the gland, focal areas of reduced echogenicity, strongly echogenic foci, accentuation of lobular architecture, and cavities. Although these changes might not be picked up when EUS is used prospectively [15, 100], Lees [ 101] concluded that some of these EUS findings are characteristic only of chronic pancreatitis. If other imaging techniques fail to provide a diagnosis, EUS may add the critical information to diagnose chronic pancreatitis. When chronic pancreatitis has been diagnosed by other methods, however, EUS is currently unable to reliably differentiate focal chronic pancreatitis from carcinomas. Although EUS is at times very helpful, this is still a diagnostic problem that requires several imaging tests and even laparotomy. In known calcific pancreatitis, shadowing makes EUS image interpretation complex.
EUS can assist in diagnosis and management of pancreatitis by differentiating pancreas divisum from a cut- off, or by documenting a small cyst that is not apparent on other imaging studies. EUS has also been used to improve the safety of endoscopic drainage of pseudocyst through the gastric wall [102]. Studies on benign tumors, strictures, and anomalous connections of the biliary sys tent are limited [21].
As mentioned previously, EUS is very accurate in detecting common bile duct stones; however, ERCP is as accurate. Because stones can be removed easily after endoscopic sphincterotomy, EUS will have a limited role in detecting common bile duct stones unless ERCP is contraindicated.
Currently, EUS has no role in diagnosis of acute pancreatitis. In relapsing pancreatitis, EUS may find a cause such as a small tumor, stone, or anomaly, that was no evident on other imaging studies.
Conclusion
EUS provides an important link between the TNM staging method and multimodal selective treatment concepts. EUS is capable of providing unsurpassed GI disease characterization and TNM tumor staging. EUS is also unsurpassed in determining resectability of GI neoplasms. EUS accuracy will continue to increase with the evolution of EUS image interpretation, as well as equipment improvements. The TNM system was created to determine prognosis and evaluate results of various therapies for tumors. The accurate TNM staging provided by EUS will allow further advances in treatment to be applied selectively. Clinical outcome of patients with GI tumors will thus improve.
The author thanks Laurel Kiefer for editorial and graphic assistance.
References
1. Snady H. Endoscopic ultrasonography: an effective new too for diagnosing gastrointestinal tumors. Oncology 1992;6:63-74
2. Behahrs 0, Henson D, Hutter R, eds. Manual for staging of cancer, ed 3. American joint Committee on Cancer. Philadelphia: J.B. Lippincott, 1988
3. Hermanek P, Sobin L, eds. International Union Against Cancer (UICC): TNM classification of malignant tumors, ed 4 Berlin: Springer-Verlag, 1987
4. DeVita VT, Hellman S, Rosenberg SA. Cancer, principle and practice of oncology. Philadelphia: J.B Lippincott, 1989
5. Forastiere AA, Orringer MB, Perez-Tamayo C, et al. Concurrent chemotherapy and radiation therapy followed by transhiatal esophagectomy for local-regional cancer of the esophagus. J Clin Oncol 1990;8:119-127
6. Hilgenberg AD, Carey RW, Wilkins EW Jr, et al. Preoperative chemotherapy, surgical resection, and selective postoperative therapy for squamous cell carcinoma of the esophagus. Ann Thorac Surg 1988;45:357-363
7. Wilke H, Preusser P, Fink U, et al. Preoperative chemotherapy locally advanced and nonresectable gastric cancer: a phase 11 study with etoposide, doxorubicine, and cisplatin. J Clin Oncol 1989;7:1318-1324
8. Snady H, Bruckner H, Siegel J, et al. Computed tomography (CT), endoscopic ultrasound (EUS) and surgical findings following combined chemotherapy and radiotherapy (CMT) to downstage unresectable pancreatic carcinoma (abstract). Am J Gastroenterol 1993;88:1542
9. Bruckner H, Kalnicki S, Dalton J, et al. Survival after combined modality therapy for pancreatic cancer. J Clin Gastroenterol 1993;16:199-203
10. Mendenhall WM, Scuba WW, Bland KL, et al. Preoperative irradiation and surgery for initially unresectable adenocarcinoma of the rectum. Am Surg 1992;58:423-429
11. Tio TL, Tytgat GNJ. Atlas of transintestinal ultrasonography. Aalsmeer, The Netherlands: Smith, Kline & French (b.v./ Mur-Kostler-voren, Gegenens Koninkligke Bibliotheek Den Haag), 1986
12. Strohm WD, Classen M. Endoscopic ultrasonography. In: Sivak M, ed. Gastroenterologic endoscopy. Philadelphia: W.B. Saunders, 1987:182-202
13. Kawai K, ed. Endoscopic ultrasonography in gastroenterology. Tokyo, Japan: Igaku-Shoin, 1988
14. Caletti GC, Barbara L. Endosonography. In: Cotton P, Tytgat G, Williams C, eds. Annual of gastrointestinal endoscopy. London, UK: Cur-rent Science Ltd., 1990:51-58
15. Rosch T, Classen M. Gastroenterological endosonography (textbook and atlas). New York: Thieme Medical Publishers, 1992
16. Lightdale C, ed. Endoscopic ultrasonography. Gastrointest Endosc Clin North Am 1992;2:557-749
17. Rosch T, Dittler HJ, Fockens P, et al. Major complications of endoscopic ultrasonography: results of a survey of 42105 cases (abstract). Gastrointest Endosc 1993;39:370
18. Caletti G, Bolondi L, Zaani L, Labo G. Technique of endoscopic ultrasonography investigation: esophagus, stomach, and duodenum. Scand J Gastroenterol 1986;21(suppl 123):1-5
19. Caletti G, Gerrari A, Barbara L. Normal endosonographic anatomy of the esophagus and stomach. Gastrointest Endosc Clin North Am 1992;2:601-614
20. Snady H. Endoscopic ultrasonography images of the normal retroperitoneum. Gastrointest Endosc Clin North Am 1992;2:637-655
21. Dancygier H. Endosonographic evaluation of biliary tract disease. Gastrointest Endosc Clin North Am 1992;2:697-714
22. Wiersema MJ, Hawes RJ. Normal colorectal anatomy and benign colon lesions. Gastrointest Endosc Clin North Am 1992; 2:715-727
23. Hildebrandt U, Feifel G. Endosonography in the diagnosis of lymph nodes. Endoscopy 1993;25:243-245
24. Heinz A, Mildenberger P, Georg M, et al. Endoscopic ultrasonography in the diagnosis of regional lymph nodes in esophageal and gastric cancer-results of studies in vitro. Endoscopy 1993;25:231-235
25. Takemoto T, Tada M, Dittler HJ, et al. Impact of staging on treatment of gastric carcinoma. Endoscopy 1993;25:46-50
26. Caletti GC, Brocchi E, Ferrari A, et al. Guillotine needle biopsy as a supplement to endosonography in the diagnosis of gastric submucosal tumors. Endoscopy 1991;23:251-254
27. Wiersema M, Hawes R, Tao L-C, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc 1992;38:35-39
28. Snady H. Combined endoscopic ultrasound and guillotine needle biopsy in the diagnosis of submucosal tumors of the gastrointestinal tract (abstract). Am J Gastroenterol 1993;88: 1599
29. Vilmann P, Hancke S, Henriksen FW, Jacobsen GK. Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy 1993;25:523-527
30. Giuli R, Sancho-Garnier H. Diagnostic, therapeutic and prognostic features of cancers of the esophagus: results of the international prospective study conducted by the OESO group (790 patients). Surgery 1986;99:614-622
31. Zuccaro G Jr, Sivak MV Jr, Rice TW. Endoscopic ultrasound and the staging of esophageal and gastric cancer. Gastrointest Endosc Clin North Am 1992;2:625-636
32. Siewert JR, Dittler HJ. Esophageal carcinoma: impact of staging on treatment. Endoscopy 1993;25:28-32
33. Souquet JC, Napoleon B, Pujol B, et al. Endosonography-guided treatment of esophageal carcinoma. Endoscopy 1992; 24:324-328
34. Caletti GC, Ferrari A, Fiorino S, et al. Staging of esophageal carcinoma by endoscopy. Endoscopy 1993;25:2-9
35. Dittler Hi, Siewert JR. Role of endoscopic ultrasonography in esophageal carcinoma. Endoscopy 1993;25:156-161
36. Rosch T, Lorenz R, Zenker K, et al. Local staging and assessment of resectability in carcinoma of the esophagus, stomach, and duodenum of endoscopic ultrasonography. Gastrointest Endosc 1992;38:460-467
37. Grimm H, Binmoeller KF, Hamper K, et al. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy 1993;25:224-230
38. Lea JW, Prager RL, Bender HW. The questionable role of computed tomography in the preoperative staging of esophageal cancer. Ann Thorac Surg 1984; 19:228-232
39. Quint L, Glazer G, Orringer M. Esophageal imaging by MRI and CT: study of normal anatomy and neoplasms. Radiology 1985;156:727-731
40. Kimmey MB, Martin RW, Silverstein FE. Clinical application of linear ultrasound probes. Endoscopy 1992;24:364-,169
41. Grimm H. The blind scope. Ninth International Symposia on Endoscopic Ultrasonography. Bologna. 1993
42. Van Dan J, Rice TW, Catalano M, et al. High grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910-2917
43. Hordijk ML, Zander H, van Blankenstein M, Tilanus HW. Influence of tumor stenosis on the accuracy of endosonography in preoperative T staging of esophageal cancer. Endoscopy 1993;25:171-175
44. Kallimanis G, Gupta PK, Al-Kawas FH, et al. Endoscopic ultrasound for staging esophageal cancer with or without dilation is clinically important and safe. Gastrointest Endosc 1994;40:65 (Abstract)
45. Botet JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology 199 1; 181:419-425
46. Lightdale CJ, Botet J F, Kelsen D P, et al. Diagnosis of recurrent upper gastrointestinal cancer at the surgical anastomosis by endoscopic ultrasound. Gastrointest Endosc 1989;35: 407-412
47. Kondo D, Imaizumi M, Abe T, et al. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest 1990;98:586-593
48. Tio TL, Blank LECM, den Hartog Jager FCA, et al. Endosonography in the clinical TNM staging and follow-up after combined radiotherapy of inoperable esophageal carcinoma (abstract). Gastrointest Endosc 1991;37:242
49. Ziegler K, Zimmer T. The role of endoscopic ultrasonography in esophageal motility disorders. Endoscopy 1992;24: 338-341
50. Botet JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology 1991;181:426-432
51. Dittler HJ, Siewert JR. Role of endoscopic ultrasonography in gastric carcinoma. Endoscopy 1993;25:162-166
52. Smith JW, Brennan MF, Botel JF, et al. Preoperative endoscopic ultrasound can predict the risk of recurrence after operation for gastric carcinoma. J Clin Oncol 1993; 11:23802385
53. Fein J, Gerdes H, Karpeh M, et al. Overstaging of ulcerated gastric cancers by endoscopic ultrasonography (abstract). Gastrointest Endosc 1993;39:274
54. Tada M, Murakami A, Karita M, et al. Endoscopic resection of early gastric cancer. Endoscopy 1993;25:445-450
55. Souquet JC, Napoleon B, Pujol B, et al. Echoendoscopy prior to endoscopic resection of gastrointestinal tumors- more safety? Endoscopy 1993;25:475-481
56. Schuder G, Hildergrandt U, Treibler D, et al. Role of endosonography in the surgical management of non-Hodgkin's lymphoma of the stomach. Endoscopy 1993;25:509-512
57. Caletti G, Barbara L. Gastric lymphoma: difficult to diagnose, difficult to stage? Endoscopy 1993;25:528-530
58. Fischbach W, Bohm S. Options in the therapy of gastric lymphoma. Endoscopy 1993;25:531-533
59. Seifert E, Schulte F, Weismuller J, et al. Endoscopic and bioptic diagnosis of malignant non-Hodgkin's lymphoma of the stomach. Endoscopy 1993;25:497-501
60. Palazzo L, Roseau G, Ruskone-Four-mestraux A, et al. Endoscopic ultrasound in the local staging of primary gastric lymphoma. Endoscopy 1993;25:502-508
61. Salles G, Herbrecht R, Tilly H. Aggressive primary gastrointestinal lymphomas. Review of 91 patients treated with the LNH-84 regimen. Am J Med 199 1;90:77-84
62. Gobbi PG, Dionogi P, Barbieri F, et al. The role of surgery in the multimodal treatment of primary gastric non-Hodgkin's lymphomas-a report of 76 cases and review of the literature. Cancer 1990;65:2528-2536
63. Fisher RI, Gaynor ER, Dahlberg ST, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med 1993;328:1002-1006
64. Andriulli A, Recchia S, Brunetto M, et al. Endoscopic ultrasonographic study of precancerous gastric lesions (abstract). Gastroenterology 1988;94:8
65. Tio TL, Tytgat GNJ. Endoscopic ultrasonography of an arteriovenous malformation in a gastric polyp. Endoscopy 1986;18:156-158
66. Rosch T, Classen M. Endosonography -what are the limits in gastroenterological diagnostics? Endoscopy 1991;23:144146
67. Yamao K. Gastric ulcer. The role of EUS in monitoring treatment. Ninth International Symposium on
68. Caletti GC, Brocchi E, Ferrari A, et al. Value of endoscopic ultrasonography in the management of portal hypertension. Endoscopy 1992;24:342-346
69. Boyce GA. Colorectal cancer staging. Gastrointest Endosc Clin North Am 1992;2:729-744
70. Izbicki JR, Blochle C. Colorectal carcinoma: impact of staging on surgical treatment. Endoscopy 1993;25:117-124
71. Herrera-Ornelas L, Justiniano J, Castillo N, et al. Metastases in small lymph nodes from colon cancer. Arch Sing 1987;122:1253-1256
72. Rikfin MD, Ehrlich SM, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal. US and CT. Radiology 1989;170:319-322
73. Rosch T, Lorenz R, Classen M. Endoscopic ultrasonography in the evaluation of colon and rectal disease. Gastrointest Endosc 1990;36(suppl):33-39
74. Shimizu S, Tada M, Kawai K. Value of endoscopic ultrasonography in the assessment of inflammatory bowel diseases Endoscopy 1992;24:354-358
75. Hildebrandt U, Kraus J, Echer KW, et al. Endosonographic differentiation of mucosal and transmural nonspecific inflammatory bowel disease. Endoscopy 1992;24:359-363
76. Appleman HD, Helwig EB. Gastric epithelioid leiomyoma and leiomyosarcoma (leiomyoblastoma). Cancer 1976;38:708 728
77. Kelsey PJ, Warshaw AL. EUS: an added test or a replacement for several? Endoscopy 1993;25:179-181
78. Snady H. Clinical utility of endoscopic ultrasonography for pancreatic tumors. Endoscopy 1993;25:182-184
79. Snady H, Cooperman A, Siegel J. Assessment of vascular involvement by pancreatic disease-a comparison of endoscopic ultrasonography to computerized tomography and angiography (abstract). Gastrointest Endosc 1990;36:197
80. Snady H, Bruckner H, Siegel J, et al. Endoscopic ultrasonographic criteria of vascular invasion by potentially resectable pancreatic tumors. Gastrointest Endosc 1994 (in press)
81. Yasuda K, Mukai H, Fujimoto S, et al. The diagnosis o pancreatic cancer by endoscopic ultrasonography. Gastrointest Endosc 1988;34:1-8
82. Edmundowicz SA, Aliperti G, Middleton WD. Preliminary experience using endoscopic ultrasonography in the diagnosis of choledocholithiasis. Endoscopy 1992;24:774-778
83. Berk J, Meshinpour H, Schapiro M, Mason G. Choledocho-lithiasis-clinical aspects. In: Berk J, Haubrich W, Kalser M, et, al, eds. Gastroenterology, vol VI, ed 4. Philadelphia: W.B
Saunders, 1985:3693-3712
84. Snady H, Cooperman A, Siegel J. Endoscopic ultrasonography compared with computed tomography with ERCP in patients with obstructive jaundice or small peripancreatic mass Gastrointest Endosc 1992;38:27-34
85. Rosch T, Lorenz R, Briag C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc 1991;37 347-352
86. Rosch T, Classen M. Endosonography -what are the limit in gastroenterological diagnostics? Endoscopy 1991;23:144 146
87. Rosch T, Braig C, Gain T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Gastroenterology 1992;102:188-199
88. Rosch T, Lightdale CJ, Botet JF, et al. Endoscopic localization of pancreatic endocrine tumors. N Engl J Med 1992;326 1721-1726
89. Palazzo L, Roseau G, Salmeron M. Endoscopic ultrasonography in the preoperative localization of pancreatic endocrine tumors. Endoscopy 1992;24:350-353
90. Rosch T, Classen M. Preoperative localization of endocrine tumors of the pancreas. Gastrointest Endosc Clin North Am 1992;2:685-696
91. Frucht H, Norton JA, London FF, et al. Detection of duodenal gastrinomas by operative endoscopic transillumination. Gastroenterology 1990;99:1622-1627
92. Tio LT. Endosonography in diagnosing and staging of pancreatic and ampullary cancer. Gastrointest Endosc Clin North Am 1992;2:673-685
93. Karl RC, Carey LC. Impact of staging on treatment of pancreatic and ampullary cancer. Endoscopy 1993;25:69-74
94. Tio TL, Reeders JWA, Sie LH, et al. Endosonography in the clinical staging of Klatskin tumor. Endoscopy 1993;25: 81-85
95. Kremer B, Henne-Bruns D, Vogel 1, et al. Impact of staging on treatment of biliary carcinoma. Endoscopy 1993;25: 92-99
96. Rosch T, Lorenz R, Braig C, Classen M. Endoscopic ultrasonography in diagnosis and staging of pancreatic and biliary tumors. Endoscopy 1992;24:304-308
97. Lees WR. The pancreas: differential diagnosis and pancreatitis. Gastrointest Endosc Clin North Am 1992;2:657-672
98. Yasuda K, Mukai H, Nakajima M, Kuwai K. Clinical application of ultrasonic probes in the biliary and pancreatic duct Endoscopy 1992;24:370-375
99. Zuccaro G Jr, Sivak V Jr. Endoscopic ultrasonography in the diagnosis of chronic pancreatitis. Endoscopy 1992;24:347 349
100. Rosch T, Lorenz R, Neuthau H, et al. The value of endoscopic ultrasound in chronic pancreatitis (abstract). Gastrointest Endosc 199 1;37:254-255
101. Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol 1986;21(suppl 123):123-129
102. Grimm H, Binmoeller KF, Soehendra N. Endosonography - guided drainage of a pancreatic pseudocyst. Gastrointest Endosc 1992;38:170-171