Pre-operative assessment of a potentially resectable peri-pancreatic mass by computed tomography (CT) is widely used, but often of limited value for lesions less than 5 cm. ERCP is frequently used to evaluate those patients with associated obstructive jaundice. To determine the clinical effectiveness of endoscopic ultrasonography (EUS), patients with pancreatobiliary lesions of less than 5 cm with or without obstructive jaundice were evaluated. CT scan, ERCP, and EUS were performed on 60 patients with a peri-pancreatic mass and/or obstructive jaundice. The results of the examinations were compared with respect to detection of an abnormality, diagnosis, and prediction of resectability. ERCP and EUS were the most sensitive and specific in detecting an abnormality of the pancreatobiliary system. The accuracy of EUS compared with the accuracy of the combination of CT scan with ERCP was significantly higher for the evaluation of the specific type and extent of pancreatobiliary disease (73% vs. 30%, p < 0.001) and prediction of resectability (75% vs. 38%, p < 0.05). EUS aided patient management in 75% by providing more details about the disease, and changed management in 32% by making a diagnosis or changing an incorrect diagnosis. EUS represents a significant advance in the evaluation and clinical management of pancreatobiliary disease. (Gastrointest Endosc 1992;38:27-34)
When a patient presents with a clinical picture suggestive of pancreatic carcinoma, standard initial evaluation usually consists of transcutaneous ultra-sonography and/or a CT scan. However, the sensitivity and specificity of these two tests vary according to the size of the mass. 1-4 A direct relationship exists between mass size and the effectiveness of these tests for detection, staging, and assessment of resectability. In those patients with small lesions, in which resection is generally considered to be the best palliation and offers the best chance of improved survival, 5,6 pre operative evaluation is the most difficult. For many patients with a peri-pancreatic mass and/or obstructive jaundice, treatment, and even diagnosis, before laparotomy is still in question after extensive evaluation with ERCP, angiography, magnetic resonance imaging, and fine needle biopsy.
Figure 1
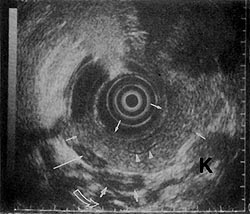
Figure 2
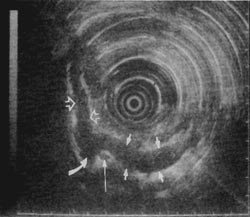
In recent years, instruments used for endoscopic ultrasonography (EUS) have evolved to be very sensitive and specific for evaluating pancreatic disease.4,7-10 Figures 1 and 2 show the precise imaging of the pancreas and portal vessels that is possible with EUS. Because of the apparent clinical utility of EUS in the evaluation of pancreatobiliary disease, we designed a study to evaluate whether EUS provides additional information to the combined findings of CT scan with ERCP, which can aid in the management of a peri-pancreatic mass and/or obstructive jaundice. Patients entered into the study had relatively small lesions that were potentially resectable.
PATIENTS AND METHODS
Sixty patients entered into the study were diagnostically problematic. All had symptoms related to pancreatobiliary disease, but had either no diagnosis after conventional studies, with laparotomy as the next planned step, or probable malignancy with uncertain tumor stage or resection status. All patients were fully ambulatory, without other significant medical disease, and were good operative risks, excluding age. No patient had evidence of metastatic carcinoma.
From May 1988 to February 1990, 39 men and 21 women (ages 28 to 92 years), were evaluated. All had been evaluated by ERCP and/or CT scan. Most patients had a standard transcutaneous sonogram that showed dilated bile ducts and/or a possible abnormality of the pancreas. Eleven of the patients had obstructive jaundice of unclear etiology; 32 patients had either obstructive jaundice or an abnormal pancreatic duct with an associated pancreatic mass of less than 5 cm on CT scan; 11 patients had pain with a pancreatic mass of less than 5 cm; and 6 patients had pain with an abnormal pancreatogram.
Techniques
CT scan with oral and intravenous contrast was performed using standard technique and scanners described by Freeny et al.11 and Zeman et al. 12 ERCP was performed in the standard manner using the Olympus JF10 or JFV10 video side-viewing endoscope. If ducts could not be visualized using standard catheters, the guidewire technique was used which improves successful cannulation to over 95%.13 EUS was performed using the Olympus GF-UM2 7.5-MHz system with a video recorder. The endoscopic part of EUS was performed similar to routine upper gastrointestinal endoscopy in an office setting. After topical pharyngeal anesthesia and intravenous premedication (meperidine and diazepam or midazolam), the GF-UM2 endoscope was introduced to the duodenum with the patient in the left lateral decubitus or prone position. Ultrasonographic scanning was then performed and recorded. A standard endoscopy with a 12.6-mm diameter scope was performed initially in 49 of 60 patients to facilitate entering the third part of the duodenum. With this technique and routine 6-month maintenance, no EUS equipment failures occurred. After the EUS scanning was completed, the scanning image could be replayed for further analysis.
The EUS scanning method employed was the balloon contact method combined with the direct water infusion method. A balloon on the tip of the endoscope is filled with 5 to 15 ml of deaerated water in conjunction with infusion of 100 to 800 ml of deaerated water through the endoscope into the stomach. Standard scanning images and optimal EUS positions according to Yasuda et al. 4 for investigating pancreatobiliary disease as well as their criteria to determine whether a lesion was malignant or benign were used.
Study protocol
CT scan was performed on all patients. ERCP was performed on all but six: three patients with unequivocal findings on other tests refused the ERCP, one was allergic to the dye, and in two others, stenosis prevented passage of the endoscope to the papilla. EUS was performed with knowledge of ERCP and CT scan findings.
Following EUS, the CT scan of each patient was reviewed by an independent board-certified radiologist to determine whether the original CT scan was technically well performed or whether the interpretation could have been more precise. After this review, seven patients were referred for a repeat dynamic CT scan using a 5-mm thin slice method similar to that described by Zeman et al.12 for the most precise CT
evaluation possible. EUS findings were then compared with combined findings of CT scan with ERCP. To most accurately reflect a typical clinical condition, the findings of CT scan and ERCP were combined so as to arrive at the most likely diagnosis to a specific diagnostic question.
Table 1
Patient data
| Disease | N | Mean age | Age range | Method of vericfication of diagnosis |
||
| Surgery | Biopsy | Clinical | ||||
| Malignant | 42 | 68 | 31-92 | 22 | 9 | 11 |
| Benign | 18 | 48 | 28-63 | 10 | 1 | 7 |
Diagnosis was verified primarily through surgery and/or biopsy (Table 1). Angiography was performed in 12. In 18 patients, verification was made through clinical course, including the performance of all tests necessary to prove progression of malignant disease or regression of benign disease. Follow-up time was a minimum of 6 months, with a mean of 13.6 months. Resectable tumors were defined as those confined to the pancreas and/or distal common bile duct in the absence of both distant metastasis and invasion of large vessels or extension into contiguous organs. Chisquare and Fisher's exact tests were used for statistical calculations.
RESULTS
Visualization of the area of disease was achieved in more than 90% for all tests (CT scan, 97%; ERCP, 93%; and EUS, 98%). No complications occurred during performance of any of the procedures. For ERCP, there were three patients in whom the common bile duct could not be visualized. The common bile duct was normal in 12, abnormal in 39. The pancreatic duct was not visualized in 17, was normal in 11, and abnormal in 26. For EUS, the area of disease was visualized in all but one patient. The level of insertion of the EUS endoscope was past the ampulla into the third/fourth part of the duodenum in 50 of 60 patients, only up to the duodenal bulb area in 6 of 60 patients, and only up to the pylorus in 4 of 60 patients. A 12.6-mm straight-viewing endoscope did not pass into the distal duodenum in 8 of the 10 patients in which the GF-UM2 endoscope did not pass, even though a 9-mm endoscope had been passed beyond the ampulla in all but one patient. The sizes of the peri-pancreatic lesions according to EUS were duct stricture, no mass: 6 patients, less than 2 cm: 14 patients, 2 to 3 cm: 15 patients, 3 to 4 cm: 18 patients, 4 to 5 cm: 7 patients.
Table 2
Detection rate for any abnormality
| Sensitivity (%) |
Specificity (%) |
|
| ERCP | 94 | 100 |
| CT Scan | 71a | 25 |
| CT Scan with ERCP | 95 | 25 |
| EUS | 98 | 100 |
Sensitivity was compared by evaluating each of the three tests in terms of its ability to detect any abnormality of the pancreas or biliary system, such as dilated or strictured ducts, or a mass. Results are shown in Table 2. ERCP alone and EUS alone were both highly sensitive and 100% specific in picking up any abnormality. CT scan, however, had the most false positives (three cases) and false negatives (sixteen cases), resulting in the lowest sensitivity and specificity. This consequently lowered the 100% specificity for ERCP alone to 25% when ERCP findings were combined with findings on CT scan. There were no false positives for ERCP or EUS.
Table 3
Ability to differentiate malignant from benign pancreatobiliary lesionsa
| CT scan with ERCP (%) |
EUS (%) |
|
| Accuracy | 72 | 83 |
| Positive predictive value | 81a | 89 |
| Negative predictive value | 57 | 79 |
| Sensitivity | 75 | 85 |
| Specificity | 65 | 80 |
We then compared the ability of the various tests to determine whether a visualized lesion was benign or malignant. Results are shown in Table 3. When malignancy was defined as a positive test and benign as a negative test, the accuracy of EUS was 83%. EUS was correct in 89% of patients predicted to have malignant disease and in 73% of those patients predicted to have benign disease, with a sensitivity and specificity for malignancy of 85% and 80%, respectively. The values for combined CT scan with ERCP were all lower. CT scans which were equivocal were re-evaluated by independent radiologists who assigned the most likely diagnosis. In retrospect, findings on three EUS examinations were diagnostic, but were initially interpreted incorrectly. If the correct interpretation had been used, the differences between EUS and combined CT scan with ERCP would have been statistically significant (p < 0.05).
Table 4
Ability to predict specific diagnosis
| CT Scan with ERCP (%) |
EUS (%) |
p | |
| All patients (N=60) | 30 | 73 | <0.001 |
| Surgical patients (N=32) | 20 | 50 | <0.05 |
Table 4 shows EUS accuracy in making a specific diagnosis as well as evaluating the extent of pancreatobiliary disease beyond differentiating malignant from benign disease. EUS was correct about twice as often as combined findings of CT scan with ERCP. This held true whether all patients were analyzed (p < 0.001), including those with diagnosis confirmed by clinical course, or whether only those undergoing laparotomy were analyzed (p < 0.05). Of those patients with benign disease, pancreatitis was found in 12, a pancreatic pseudocyst in 3, pancreatic stones in 1, and vascular anomalies in 2. Of those with malignant disease, using the American Joint Committee on Cancer TNM system for describing anatomical extent of disease, 10 had T1 or T2 disease, 15 had T3 disease, and 17 had distant metastasis.
Table 5
Pre-operative assessment of resectability
| CT scan with ERCP (%) |
EUS (%) |
p | |
| Accuracy | 38 | 75 | 0.05 |
| Positive predictive value | 40 | 67 | Nsa |
| Negative predictive value | 5 | 89 | 0.05 |
| Sensitivity | 73 | 91 | NS |
| Specificity | 8 | 62 | 0.01 |
Table 5 shows a higher accuracy of EUS compared with CT scan for pre-operative assessment of resectability. Only those 24 patients with an initial CT scan showing a probable resectable tumor who then went on to laparotomy were included for analysis. A later review of CT scan showed evidence of unresectability in one patient. Ten other patients, not included for analysis, had a CT scan showing a resectable tumor, an EUS showing unresectable disease, and did not have a laparotomy. Five of these 10 were confirmed to be unresectable after 1 to 2 months on follow-up tests. If these 10 patients are included, then the difference in accuracy between EUS (82%) and CT scan (26%) would be even greater. Six errors in EUS were as follows. In two patients local extension into surrounding organs and in two other patients liver metastasis were missed on all preoperative tests. In two patients EUS findings were misinterpreted, but in retrospect should have predicted resection status accurately. For CT scan, there were 15 errors, 8 of which were in assessing venous involvement. For assessing resectability there was no case where EUS was not accurate when CT scan was accurate.
When we looked at the additional information provided by EUS compared with combined CT scan with ERCP in terms of clinical effectiveness, we found that EUS aided clinical management by providing more detail about a disease in 75% of all patients. The information provided by EUS alone was as follows: (1) normal pancreas; disease found elsewhere on further work-up: four patients; (2) benign chronic pancreatitis with or without acute disease; no further work-up required: five patients; (3) non-resolving benign disease demonstrated; surgical treatment successful: four patients; (4) surgical approach modified based on EUS findings: eight patients; (5) carcinoma confined to pancreatic parenchyma as etiology of a biliary stricture: 6 patients (two confirmed at resection, four managed non-operatively because age of patient over 75 years); and (6) carcinoma unresectable because of vascular involvement: 15 patients, or metastatic disease: 3 patients.
EUS completely changed the course of clinical management in 32% of all patients by either making a diagnosis or changing an incorrect CT scan/ERCP diagnosis or tumor stage. Of the 20 patients where EUS changed clinical management, EUS detected false positives in 6 and false negatives in 13. False positives were defined as disease that an examination found to be more extensive than the actual pathology; false negatives were defined as disease that an examination found to be less extensive than the actual pathology. The scale was comprised of six categories: no disease, benign resolving disease, benign non-resolving disease, malignant localized disease, malignant invasive disease, and metastatic disease. Management plan changed for the following reasons:
1. EUS established diagnosis of benign pancreatic disease resulting in appropriate therapy in seven patients (surgery in three, no surgery required in four).
2. On conventional pre-operative evaluation, malignancy appeared to be unresectable. EUS demonstrated focal disease confined to the pancreas which was resected in two patients. No evidence of recurrence was noted after a mean of 12 months.
3. On conventional pre-operative evaluation, probable malignancy appeared to be resectable. EUS demonstrated unresectable malignancy in 10 patients.
Figure 3
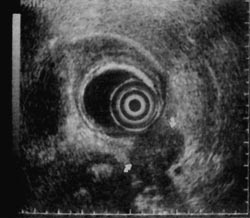
Figure 4
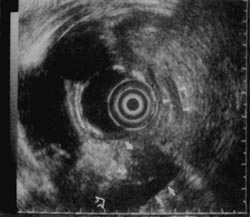
Figure 5
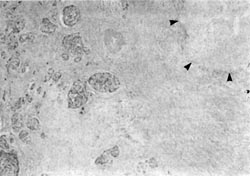
Figure 6
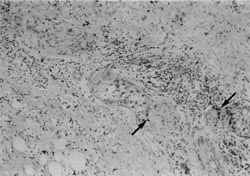
An example of both the utility and limitation of EUS is illustrated in Figures 3 to 6. A 43-year-old man with abdominal pain who on CT scan had mild enlargement of the pancreas, but no focal mass, and on ERCP had a cutoff of the pancreatic duct possibly representing pancreas divisum was examined. He had had a laparotomy 2 years earlier with the diagnosis of acute pancreatitis. EUS showed (Fig. 3) that the body of the pancreas was not homogenous. There was a well-defined, round, smooth-edged, mottled, hypoechoic area approximately 2-cm in diameter, which was interpreted as a localized area of chronic pancreatitis not resolving for unclear reasons. The pancreatic duct in the tail was dilated to 5-mm behind the mass (Fig. 4). In addition, the duct was echogenic, suggesting proteinaceous material in the obstructed duct. Since EUS demonstrated a localized lesion, EUS aided management. A resection was recommended rather than the usual drainage procedure. Histology of the resection specimen showed chronic pancreatitis with an obstructed duct (Fig. 5). In Figure 6, the reason why the pancreatitis had not resolved was evident; in one part of the pancreas, there was peri-neural invasion by malignant cells. For this patient, CT scan, ERCP, and EUS all were sensitive and specific in detecting an abnormality, but were incorrect in diagnosing benign disease.
Figure 7
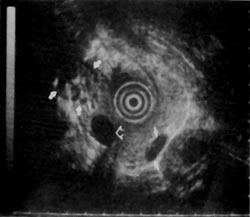
Figure 8

Figure 9
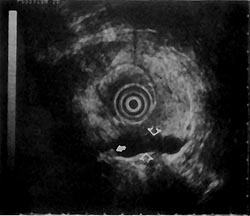
An example of a false positive in which EUS changed the course of clinical management is as follows. A 67-year-old man presented with what appeared on CT scan to be an unresectable pancreatic mass originating from the uncinate and causing common bile duct obstruction. ERCP confirmed the presence of a common bile duct stricture. EUS of the pancreas showed a hypoechoic area in the superior part of the head of the pancreas near the portal vein. The hypoechoic area was approximately 1-cm with rough margins, but was contained by fibrosis appearing as highly echogenic foci in the surrounding tissue (Fig. 7). Resection was therefore recommended. The resection specimen showed a normal uncinate and a desmoplastic reaction surrounding a focal carcinoma that was completely resected. A follow-up examination 14 months after surgery showed no evidence of recurrent disease.
Another example where EUS changed the course of clinical management is that of a false negative. A 70-year-old woman with obstructive jaundice was found on cholangiography to have a stricture at the bifurcation. Standard transcutaneous sonography and CT scan showed a possible mass in the left lobe of the liver. Endoscopic, percutaneous, and fine needle biopsies of the area were all negative for malignancy. Magnetic resonance imaging appeared similar to the CT scan, indicating a probable lesion in the left lobe of the liver. However, magnetic resonance imaging gave no additional information about the portal venous system. Part of the hepatic vein was seen, but the portal vein was not visualized. On EUS, the portal vein was visualized (Fig. 8). Figure 9 shows another angle on the portal vein from which invasion was clearly seen, not only establishing the diagnosis of malignancy, but also the unresectability of the lesion. In addition, EUS demonstrated other areas of probable metastatic disease of the liver. Although surgery was being considered before EUS, the patient was subsequently managed with biliary endoprostheses.
DISCUSSION
In 1980, EUS was developed to evaluate digestive organs. 14-16 Because of the promising results of this technique, 4,17,18 we used EUS to evaluate patients with pancreatobiliary lesions less than 5-cm, with or without obstructive jaundice and no evidence of metastatic tumor. The study was conducted to determine the clinical effectiveness of information provided by EUS beyond that provided by CT scan and ERCP in a subgroup of patients in whom laparotomy would have been the next diagnostic test.
The present study found that EUS changes clinical management in a significant one third of cases and represents a major advance in evaluation of pancreatobiliary disease. In patients with lesions less than 5-cm and difficult diagnostic problems following an abnormal ERCP or CT scan, EUS was more accurate, sensitive, and specific compared with combined findings of CT scan with ERCP for detection of an abnormality, for diagnosis, and for prediction of resectability. Surprisingly, combining CT scan findings with those of ERCP reduced the accuracy of ERCP alone in detecting pancreatobiliary disease. The specificity of CT scan seems particularly low compared with other reports' because the group of patients we studied had small lesions. Data demonstrating the high accuracy of CT scan for all stages of disease' has been assumed to equally apply to patients with small lesions.
Compared with EUS, a successful ERCP provided superior imaging of the common bile duct and pancreatic duct. However, when ERCP did not visualize a part of the pancreatic duct or common bile duct, this area was usually seen on EUS. In several studies, ERCP has been shown to be able to differentiate benign from malignant disease in about 75%.4,6,19 Because patients in our study were referred with difficult diagnostic problems, ERCP was only able to make this differentiation in 50%. Although ERCP alone was quite accurate in detecting pancreatobiliary disease, the limited information which ERCP provides about surrounding parenchyma and blood vessels reduces its utility in evaluation of the extent of disease. CT scan information about pancreatic parenchyma for small lesions is also limited. 5,20
Of the present conventional methods for diagnosis of small pancreatic lesions, ERCP appears to be the most reliable, detecting 60 to 80%, depending on size.6,19 For many patients with obstructive jaundice, CT scan or transcutaneous sonography cannot visualize a lesion which on ERCP appears to be small and in the distal common bile duct or head of the pancreas. Accurate determination of tumor stage by CT scan or sonography is obviously low for these lesions. 20 Warshaw et al.5 found CT scan to be correct in predicting resectability in only 45%. This value is similar to our finding of 38% accuracy for those patients who underwent laparotomy (Table 5). Some small lesions in the pancreas cannot be detected even by ERCP. EUS is able to diagnose small tumors less than 2-cm not found by other imaging techniques. No improvement in CT scan evaluation of small pancreatic tumors was observed in those cases that had a repeat CT scan with 5-mm thin slices. Tio et al. 21 have reported EUS to be accurate in pre-operative classification of ampullopancreatic carcinoma with a 66% staging accuracy and 92% accuracy for tumor invasion. The EUS findings were not compared with other preoperative tests, however, since their objective was to correlate EUS findings to histopathology.
Limitations of EUS at the current state of technology include: (1) The optimal focal range is only 2 to 4 cm from the probe. Therefore, significant portions of the right lobe of the liver cannot be visualized. Also, with tumors larger than 5-cm, optimal focus of nearby vessels may not always be achieved. These shortcomings should be overcome when EUS instruments are developed with the capability of scanning at lower as well as higher frequencies, such as from 3.5 to 15 MHz. The Olympus GF-UM3 endoscope has an additional 12 MHz frequency which improves imaging of lesions 1 to 3 cm from the transducer, but this does not improve imaging of distant tumors. (2) Although EUS is superior to other imaging techniques, the resolution of lymph nodes, particularly those less than 1 cm, and the criteria to distinguish malignant involvement still require improvement. 21 (3) The echo pattern and features of EUS images of various pancreatic diseases can appear to be quite similar; consequently, at the present time, criteria to distinguish different diseases overlap.22 As shown in our patient (Figs. 3 to 6), the current criteria 4 used to distinguish benign from malignant focal lesions on EUS require improvement. Some of this overlap is inherent to the ultrasound pulses. However, some of the overlap is a result of the limited experience with interpretation of EUS images. In our series, misinterpretation of sonographic images resulted in diagnostic errors in 5 of 60 patients. The ability to perform sonographically guided biopsies of a lesion through the GF-UM2 or GF-UM3 endoscopes would resolve many problems of image interpretation, but such capabilities are presently lacking. Transcutaneous sonographically guided core biopsy appears to be a significant improvement over fine needle biopsy.23 The evolution of EUS image interpretation as well as equipment improvements will further increase EUS accuracy.
Sensitivity and specificity of a test to evaluate tumors does not remain constant for lesions of varying size. Accuracy of standard transcutaneous sonography to evaluate pancreatobiliary disease also varies greatly due to the wide variety of instruments in use over the past 10 years.3,24 However, our results and those of others suggest that initial evaluation using newer high resolution instruments will accurately select out and evaluate pancreatobiliary tumors larger than 5-cm.24,25 EUS is unlikely to provide additional information on patients with tumors larger than 5 cm (not included in our study) because the EUS probe cannot always be brought close enough to a lesion to bring its margins into optimal focus.4
For pancreatobiliary disease, accurate CT scan interpretation relies primarily on distortions of surrounding fat planes. Thus, the natural history with large tumors makes it likely that these fat planes will be altered and CT scan will be accurate. However, patients with tumors less than 5-cm can have either weight loss with an accompanying loss of the fat planes or a small tumor not large enough to alter fat planes. Density of such tumors is often so similar to that of normal pancreas that they are undetected despite very thin CT scan slices. Thus, for lesions that on standard ultrasonography are less than 5-cm and appear to be localized, EUS will provide the information required to select the most appropriate management. 26-28
Common bile duct stones occasionally can present with a clinical impression of a tumor. They can be diagnosed by standard ultrasonography or CT scan, but usually in less than 50% of cases. Common bile duct stones appear to be reliably diagnosed by EUS in most cases.29 But, since more than 80% of common bile duct stones can be detected using clinical and laboratory criteria alone,30-34 we did not include such patients in our study. By excluding patients with common bile duct stones, our results show that EUS is significantly more accurate than CT scan to evaluate obstructive jaundice due to small tumors.
The high accuracy of real-time EUS is primarily due to its unsurpassed resolution of the parenchyma of the pancreas and its capability of evaluating and integrating mucosal, vascular, ductal, and parenchymal abnormalities caused by disease. To obtain information about these four types of abnormalities, four separate tests are often required: endoscopy for mucosa; venogram or arteriogram for veins and arteries; ERCP for ducts; and CT scan or standard transcutaneous sonography for parenchyma and lymph nodes. We found that the additional information obtained from EUS resulted in a major change of course in clinical management in one third of cases, and in three fourths of cases aided clinical decisions. The extent of reduction in morbidity, mortality, and improved quality of life that may result from the change in clinical management due to EUS findings will be determined as more patients are followed up for longer periods of time.
ACKNOWLEDGMENTS
We thank L. Kiefer for editorial assistance and C. Yeh, F. Winsberg, D. Neistadt, R. Neff, G. Halpern and B. Cohen for radiologic assistance.
REFERENCES