Artifacts and Techniques of Endoscopic Ultrasonogaphy in Investigating Gastrointestinal Pathologies and Theraputic Options
Harry Snady, M.D., Ph.D., Department of GastroenterologyMount Sinai Medical Center
New York, New York
Introduction
Attempts to combine ultrasonography (US) and endoscopy have developed to the point where endoscopic ultrasonography (EUS), also known as echoendoscopy, has become the most significant advance for imaging the gastrointestinal (GI) tract wall and contiguous organs in the past 20 years.
1-3 The detailed resolution seen on EUS images is unmatched by any other current method. 4 EUS is regarded by endoscopists as the most difficult procedure in gastroenterology. 5,6 In a survey of 21 centres representing over 12,000 EUS cases (Snady H, unpublished observations, 1993), all but one rated pancreatic EUS as at least as difficult as papillotomy with stent placement, the endoscopic procedure generally regarded as requiring the most training and skill. Thirteen of 21 centres rated EUS as more difficult. Artifacts that complicate image interpretation are to a great extent responsible for the Difficulties in mastering EUS. Nevertheless, the unsurpassed accuracy of EUS, as compared to any other imaging technique, has been established repeatedly through retrospective, prospective, in vitro and in vivo studies.7-14 Studies have been performed on normal tissues as , well as for non-operative GI tumour characterization and staging, documenting the high accuracy of TNM staging that is possible with EUS. 4 EUS can provide unique information, essential to diagnose malignancy, that is often otherwise obtainable only by surgical techniques.The number and influence of animal studies have been limited. However, advances in the clinical application of EUS depend on refining image interpretations. The study of EUS in animals should improve interpretation of sonographic images through correlation of histology of disease to sonography.
In this chapter, optimal methods for performing EUS and factors such as ultrasound image artifacts, which contribute to accurate EUS interpretation, will be reviewed and discussed. The EUS technique can then be applied to understanding mechanisms inherent to GI tissue pathologies. The applicability of EUS to clinical problems will also be addressed.
Methods
Successful performance of EUS requires both specific equipment and special technique. In addition, a basic understanding of sonographic imaging is necessary. Finally, image interpretation requires a thorough knowledge of anatomical relationships of vessels and organs.
4,7,8,10,11,15-19Equipment
Mechanical and electrical methods of ultrasound scanning have been applied to intraluminal sonography. For the mechanical Olympus UM sector scan systems (Olympus Optical Co Ltd, Tokyo, Japan), a motor rotates a single piezoelectric transducer to produce a 360" circular scan that is perpendicular to the echoendoscope shaft. This circular sector facilitates orientation of the probe in the lumen of the GI tract and is the most widely used for scanning because rapid orientation allows faster, more efficient scanning. However, biopsies are not usually performed with this instrument. Because the needle is seen only as a single point where it pierces the perpendicular ultrasound image plane, the needle is difficult to direct. The linear array sector echoendoscopy system (Pentax/ Hitachi, Pentax Precision Instruments Corp., Orangeburg, NY, USA) electronically switches an array of piezoelectric transducers to produce a linear scan image that is parallel to the endoscope shaft. Although the effective image provided is only a 105" wedge, making scanning more difficult, particularly in the oesophagus, this image orientation allows a biospy needle to be more easily directed and followed into the target because it is in line with the scanning plane.
20 Equipment has been developed to complement biopsy capabilities of the Olympus systern.A reflecting mirror converts the sector scan from perpendicular to parallel to the scope. Most echoendoscopes currently have a side or oblique angle of view with the exception of the echocolonoscope which is forward viewing. The echocolonoscope has an accessory channel with the optical components resulting in a 40' wedge cut from the full 360' EUS image. The echoendoscope can be passed into the distal duodenum, and the caecurn can be reached with the echocolonoscope.
Small 2-3.7 mm diameter probes with a frequency of up to 50 MHz have been developed which can be passed through standard endoscopes. These probes are then placed against a target area. Because of significant attenuation of high frequency sound waves, depth of resolution of these probes is <1 cm. Their clinical utility is still being defined. Probes have been developed by numerous manufacturers including: Olympus Optical Co., Aloaka Co., Machida Co., Toshiba Medical Co., Fuji Photo Optical Co., Boston Scientific Corp. and Brual and Kjaeer Medical Systems.
10,21-28Technique
As with all endoscopic techniques, passing the instrument through the lumen is relatively easy. The difficulty arises in passing the scope to the target area, identifying and examining the target, and then interpreting the images and artifacts.
EUS is performed in a fasting patient. Pharyngeal anaesthesia and intravenous sedation are generally used, but are not required. Endoscopy with a standard endoscope is performed initially because the echoendoscope has limited capabilities as an endoscope. The rigid tip and the oblique viewing optics of the echoendoscope make examination of the mucosa and the lumen more difficult than with standard endoscopes, and similar to that with a large diameter side-viewing endoscope. Initial standard endoscopy allows for assessment of endoscopic anatomy and detection of areas such as strictures that may be at risk for a complication.
During EUS scanning, the transducer is guided through the GI lumen and placed directly adjacent to the area of interest. Bones, adipose tissue and air-filled structures, which limit the sound wave imaging clarity of extracorporeal sonography, are avoided. The close proximity of the ultrasonic source to the organs imaged permits the use of high frequency, high resolution sound waves that have too short a penetration depth to be used in transcutaneous sonography. Current EUS instruments use the higher sound wave frequencies of 7.5 and 12 MHz, as compared with 3.5 MHz for transcutaneous US and 5 MHz for blind rectal and oesophageal probes.
As with all sonography, the sound wave imaging plane can be oriented at any angle to bring the lesion into optimal focus simply by turning the probe. The operator is not limited to parallel sections, usually 1cm apart, as with computed tomography (CT). When the echoendoscope is in the target area, intraluminal gas is aspirated to increase contact with the GI wall, thus maximizing available acoustic windows. The area to be imaged is brought into focus by filling a thin Latex rubber balloon (Olympus Optical Co. Ltd) surrounding the transducer at the tip of the echoendoscope with approximately 15 ml water. The water-filled balloon serves as a non-attenuating, acoustic coupling medium between the transducer and the surface of the GI wall. If the tar,et area is in the wall itself, water (up to 1000 ml) can be placed directly into the lumen for optimal acoustic coupling. The scanning plane is then manoeuvered, using scope controls and turning, pushing or pulling the scope shaft to orient the lesion into optimal position and focus. The technique will vary according to the indications arid objectives of the examination.
EUS images of normal anatomy
EUS sonographic layers of the wall of the GI tract have been correlated to histopathological layers in several studies.
4 The standard five layer EUS image of the GI tract wall and its correlation to histological layers is shown in Fig. 13.1a.16 However, additional histological layers can be seen when different factors change the sonographic resolution of the intestinal wall. When a pathological process changes sonographic interfaces, seven layers (Fig. 13. 1 b) can be seen (Snady H, unpublished observations). UP to nine layers have been observed when different frequencies are used.27,28 Optimal imaging of the GI wall is essential to determine the wall layer(s) affected by disease. With the sonographic plane oriented as closests possible to perpendicular to the intestinal wall, optimal imaging and limitation of artifacts can be achieved. Substances injected into the GI wall can be located with EUS (Snady H, unpublished observations) to demonstrate which layers of the GI tract wall are altered. (Fig. 13. 1c). EUS images of organs, large vessels and lymph nodes contiguous to the GI tract each have their own characteristic appearance from seven standard position 7 used for orientation and finding anatomical landmarks. The normal anatomy of the oesophagus, stomach, pancreas, retroperitoneurn and hepatobiliary tract have been described previously. 4,7,8,10,11,15-19Figure 13.1a
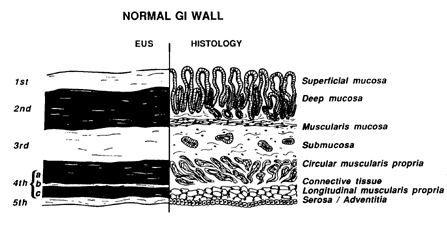
(a) Correlation between the standard five EUS layers and histological layers of the normal intestinal wall. 1st = interface between fluid in the lumen and the superficial mucosa; 2nd = lamina propria and muscularis mucosa, or deep mucosa; 3rd = submucosa and interface between submucasa and muscularis propria; 4th = muscularis propria; circular (4a) and longitudinal (4c) are not usually seen as separate layers since the thin connective tissue layer (4b) is normally not seen; 5th = interface between serosa and surrounding adventitial tissue. Reproduced from Ref. 16, with permission.
Figure 13.1b
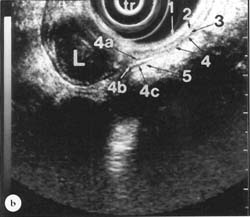
EUS image showing five layers of the gastric wall that become seven lavers near a leiomyorna (L) arising from the inner layer of the muscularis propria. 1, 3, 4b and 5 = 1st, 3rd, 5th and 7th layers are hyperechoic (white). 2, 4a and 4c = 2nd, 4th and 6th lavers are hypoechoic (black). 4b = the connective tissue layer between the 4a = inner, circular layer and 4c = outer, longitudinal layer of the muscularis propria is generally not seen in the typical five laver image. Transducer (tr) is surrounded bv a water-filled balloon. EUS, magnification range scale = 6cm.
Figure 13.1c
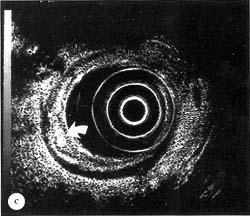
(c) Hyperechoic area (arrow) developing in deep mucosal, hypoechoic, 2nd layer of colonic wall after injection of normal saline into colonic wall with a 3-4 mm 23 gauge endoscopic injection needle (Varijet, Boston Scientific Corp., Watertown, MA, USA). EUS magnification range scale = 6 cm.
Direct correlation of EUS images to anatomy, particularly for the pancreatobiliary systern, have been difficult to perform. Consequently, precise correlation of various EUS findings to actual histopathology has been possible only through indirect methods, which rely on the analysis of pathology from operative or autopsy specimens. Although the pancreatobiliary system can be imaged in various animal models, the variations in anatomy from the human anatomy make EUS animal studies of limited value (H Snady, unpublished observations). Intraoperative EUS has also not been helpful; when the peritoneal cavity is opened, air distorts the EUS image (H Snady, unpublished observations). However, intraoperative EUS has been useful for analysis of lymph nodes.
29 An understanding of lymph nodes associated with each organ is crucial to understanding where to scan for spread of disease to regional lymph nodes.Tumour staging
The role of EUS in determining patient outcome, both in terms of prognosis and quality of life, depends upon co-ordination of EUS with meaningful pathological staging and effective treatment. As more effective treatments for GI neoplasms involving combined chernotherapy/ radiotherapy plus surgery have evolved, increased response rates and improved survival have been reported.
4,14 Even when not curative, therapy may alter the course and life history of GI cancer. Studies show that accurate preoperative staging is essential in determining the timing and dosage of specific treatments for greatest efficacy and impact.4For most cancer sites, the staging recommendations that are accepted and used worldwide are concerned only with anatomical extent of disease. An untreated primary cancer or tumour (T) progressively increases in depth of invasion, spreads to regional lymph nodes (N), and finally, as a result of continued extension of disease, metastasizes (M) to distant lymph nodes or organs. TNM classification and stage grouping is thus a method of designating the extent of a particular type of cancer as it is related to the natural course. Manuals
30,31 with recommendations for stage groupings of cancer at all anatomical sites have been published by The American joint Committee of Cancer (AJCC) and The International Union Against Cancer (UICC). These evolving recommendations of the TNM system, developed by Denoix, 32 are based on contributions from 400 expert participants over 40 years and replace other non-uniform staging methods.33.34 The TNM tumour staging method has proven to be very accurate in determining prognosis. For GI tumours, EUS is the most accurate single technique to determine stage according to the TNM system.Progress in predicting prognosis of a tumour will continue with further refinement of both the TNM system as well as the ability of EUS to predict the extent of tumour spread. Limitations of the TNM system for GI neoplastic disease have been reviewed.
35 Limitations of prediction of tumour stage with EUS are related to the overlap of current criteria used to differentiate the echo patterns and features of disease processes. Part of this overlap is inherent to the ultrasound Pulses; however, part is a result of limited experience with the subtle distinctions required for the most refined EUS interpretation that is possible. As criteria continue to be developed, clarified and conjoined, image interpretation will continue to improve.36-38 In addition, EUS will have a major role in defining abnormal areas as small as 5-10 mm for directed deep biopsies from the GI lumen through the GI tract wall. 20,39-41 Combining histology of a tissue sample with EUS imaging of the entire tumour will improve the process of differentiating neoplastic tissue from inflammation.Sonographic principles and artifacts
The display of the interaction of sound waves with tissues on a monitor is the basis of clinical US. Understanding this interaction is the primary factor in using artifacts and in achieving optimal images. Accurate clinical US image interpretation depends on understanding the properties of sound waves. the mechanics of the equipment, the characteristics of the tissue or suspension media. as well as the proper technique.
3-6,42-46 Errors in EUS can occur at any phase of image generation and interpretation.5,6,43-47 They can be divided into categories according to origin (Table 13.1). If maximum effort is not made to produce an optimal image, artifacts inherent to ultrasonography are magnified and even created, so that accurate interpretation is virtually impossible. Each of these features of EUS will be discussed to highlight aspects required to produce consistent, quality imaging. Table 13.1 - Sources of EUS errors and artifacts.- Equipment
- Malfunction or improper operation
- Improper calibration
- Imaged object out of frequecy's focal range
- Improper adjustment of electreical controls
- Acoustic presentation of the image
- Assumptions made by the sonographic instrument to produce an image
- Physical properties of the sound beam
- Acoustic properties of the tissue
- Interaction of sound and tissue
- Characteristics of the tissue or medium
- EUS technique
- Improper operation of the transducer resulting in:
- Non-perpenducular scanning
- Object compression artifacts
- Insufficient contact at appropriate anatomical acoustic window
- Misinterpretation of anatomy
- Improper focal length adjustment altering acoustic presentation of the image
- Improper operation of the transducer resulting in:
The term ultrasound refers to sound wave frequencies >20,000 Hz that are beyond human hearing. Ultrasound frequencies from 1 to 50 MHz have been investigated for clinical use. When performing US, factors that must be considered include: certain basic and necessary reductive assumptions that must be made to build an image-producing machine, the physical nature of the sound beam itself, and the acoustic properties of the objects being imaged.
43-46Absorption, reflection, refraction and scatter are behaviours of sound waves as they propagate through and interact with tissue. Through absorption the mechanical energy of the sound pulse is converted to heat. Reflection and refraction refer to the portion of sound that returns or emerges from a boundary or interface of a medium. Scattering occurs because of diffusion or redirection of sound in various angles when it encounters a particle suspension or a rough surface, resulting in sound energy not returning to the transducer and, therefore, loss of its detection by the transducer. Acoustic impedance (resistance) is the product of wave velocity through the medium and density of the medium. All biological tissue and media have inherent acoustical properties and impedance. As sound travels through a medium, it looses energy through interactions of sound and tissue, and becomes attenuated. Higher frequency sound waves are subject to a greater degree of attenuation. Spatial resolution is also related to frequency. The higher the frequency, the better the spatial resolution. Although high frequency sound waves can penetrate deeper levels, images will be out of focus when attenuation overcomes the gain in spatial resolution.
Reflection is an important property of sound waves required for image formation in US. Sound reflection is maximized when a high amplitude beam strikes a soft tissue-gas or fluid-gas interface at a perpendicular angle. When the acoustic impedance of two adjacent tissues is different, sound striking the tissue interface is reflected, producing a sound wave echo which returns to the transducer. The reflected sound wave is translated to the screen, placing the interface at a specific point in the image that correlates with the distance from the transducer to the interface in the tissue. The greater the difference in tissue impedance, the stronger the amount of reflection. Reflection is also intensified with greater amplitude of the incident beam.
Imaging artifactsImaging artifacts are misrepresentations of the true nature of the structure or tissue being displayed on a monitor. Artifacts can cause misreading of EUS images because of optical illusions, errors of interpretation, and interobserver variability related to perception and/or incorrect or incomplete definitions and criteria of various terms and findings.
36-38,42-47Equipment artifacts
There are certain artifacts which relate to equipment malfunction. These occur when air, water or dust gain access to the oil-immersed-transducer housing, the transducer, or the electrical connections. These types of artifacts are generally easy to detect as they will be present on the screen even when the instrument is not being used for scanning.
Because of attenuation, the optimal focal range of an instrument will vary with frequency. For higher frequencies, the focal length is smaller and closer to the transducer; for lower frequencies, the focal length is further away from the transducer. The optimal focal range for 7.25 MHz is 1-4 cm and for 12 MHz, 2-20 mm. Objects that are not in the focal range of the Sound beam cannot be consistently and accurately deciphered (Fig. 13.2a and b). Adjustment of image intensity with amplification (gain) or using adjustments in contrast, focal zone and depth can compensate for some of these factors in producing an accurate image. However, improper machine settings as well as improper adjustments of equipment controls during, scanning can cause artifacts.
Figure 13.2a
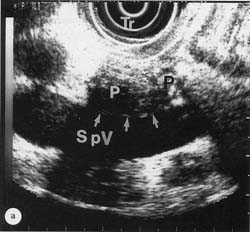
(a) 7..5 MHz EUS image of pancreas (P) with the transducer (Tr) against the posterior wall of the stomach. Hyperechoic interface (arrows) between pancreas and splenic vein (SpV) is clearly seen. EUS magnification range scale = 6cm.
Figure 13.2b
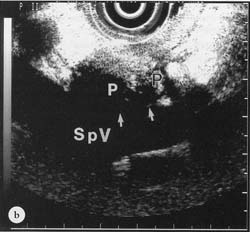
(b) 12 MHz EUS image of pancreas with transducer in exactly the same position as in (a). Hyperechoic interface between pancreas and splenic vein not clearly seen. EUS magni-fication range scale = 6 cm.
Acoustic artifacts
Artifacts of acoustic origin are pervasive throughout grey-scale imaging and are related to resolution, propagation and attenuation. This type of artifact can be grouped in terms of the effects they produce such as: added objects, missing objects, and incorrect object brightness, location, size or shape. These artifacts can be related to specific causes such as pulse length, pulse width, interference, reflection, refraction, side lobes, grating lobes, attenuation, focusing, reverberation, resonance speed error and high pulse repetition frequency.
42-46 In ultrasound, acoustic artifacts arise as a consequence of reductive assumptions when the machine produces an image, physical characteristics of the sound beam, and tissue acoustic properties. cm. (b) 12 MHz EUS image of pancreas with transducer in exactly the same position as in (a). Hyperechoic interface between pancreas and splenic vein not clearly seen. EUS magnification range scale = 6 cm.Artifacts occurring as the necessary consequence of reductive assumptions
The US instrument generates, records and processes complex signals using assumptions that are simple and consistent, but flawed in all but ideal situations. Artifacts result when significant violations of these assumptions occur. These assumed principles include: Sound always travels in straight lines; only the properties of the imaged object directly determine the intensity of returning echoes; distance is directly proportional to the time it takes for an echo to make a round trip and return along the propagation path to the surface of the transducer.
Reverberation occurs as sound strikes a subjacent interface and is reflected many times between the interface and the transducer surface. When these multiple reflections are strong enough, they are detected by the receiver and given a spatial alignment that is a multiple of the depth of the original reflective interface. These artifactual echoes occur especially within soft tissue or fluid structures, and sianificant1v alter the echo texture of the object. Reverberations can simulate disease, such as a pseudomass or thrombus in a vessel, when the additional 'non-real' reflectors are placed on the image. The water-filled balloon around the EUS transducer can also be a cause of reverberation artifacts.
Short-path reverberations cause comet tail or ring down artifacts, seen behind a small but intense reflector such as air, metal, plastic or calcified objects. They appear as a series of closely spaced echoes that trail off in intensity as distance from the object increases. The short path is probably produced by microbubbles or crystalline structures that set up reverberation chambers.
Multipath artifacts occur when the reflected sound beam maintains its intensity and coherence at flat and smooth interfaces. Acoustic noise is produced by back scattering of many secondary sound waves from surrounding tissue which reflect again off the smooth interface and return signals to the transducer surface. The result is the visible acoustic noise or dirty shadowing behind a reflecting surface such as a large, smooth calcification or gas pocket. However, the coherence of the reflected beam depends on the diffractive nature of the presenting Surface. If the insonified surface is rough and/or has a small radius of curvature, the back-scattered beam will be diffuse, producing phase incoherence in the return beam. Since absorption, not reflection, of sound wave is the dominant process, phase cancellations and loss of signal occur, which cause clean shadowing.
Mirror image artifacts occur when sound takes longer, more indirect path from the primary interface to a secondary interface before finally returning to the transducer surface. The processor assumes a straight line path and places a phantom lesion at a location deep to the primary reflector due to misregistration of some secondary reflectors. Mirror image artifacts are commonly found around the diaphragm, pleura and bowel.. Similar phenomena are seen in colour Doppler.
Side lobe artifacts (from single element transducers) and grating lobe artifacts (from arrays) result from several low-intensity side lobe sound beams around the main ultrasound beam. These side beams can interact with reflectors and present sound back to the transducer face causing objects to be displayed incorrectly in a lateral position. The instrument, believing the integrity of a single main beam, assigns these side echoes a fictitious position within the path of the main beam. Side lobe signals are most significant at highly reflective interfaces and cause the true echo texture of the imaged object to be altered by low level echoes.
Focal zone banding artifacts occur because brighter shades on the grey scale are always assigned to higher amplitude echoes. However, a sound bearn varies in amplitude along its propagation path, resulting in focal zones of increased intensity. Electronic focusing can therefore create focal zone bands of alternating high and low intensity in an organ that is actual1v homogeneous. A pseudhypoechoic mass can be created in an organ through such banding.
Flat artifact is seen with colour Doppler and has not played a significant role in EUS. It occurs when colour is suppressed where grey-scale echoes are present, but assigned to anechoic or hypoechoic areas.
Artifacts due to sound beam shape
Spatial resolution limitations call result in artifacts. Objects that are separated by a small distance call merge on the screen if the pulse length is not short enough to distinguish two closely spaced points. Axial resolution is superior to lateral resolution because pulse length is normally much less than pulse width. Axial resolution generally improves ,with higher frequencies. Lateral resolution call range up to 3 cm, whereas axial resolution is usually no more than 2-3 mm. Therefore, small objects may appear larger or thicker when reflectors are parallel to the bearn compared to those that are encountered perpendicular to the beam. Measurements are best made in the axial direction as often as possible.
Image speckle, an interference pattern close to the transducer, causes a parenchymal echopattern, but also image degradation. This acoustic noise is produced by the constructive and destructive interference of rotating echoes from a scatter distribution. Speckle call be reduced by photographic averaging and deconvolution resulting in, improved images.
Slice thickness artifact, also termed section thickness, off-axis or beam-width artifact. results in the melding of the image of different tissues that may not belong together. A focused US pulse has a finite width in the direction perpendicular to the scan plane. The beam can therefore sonographically locate more than one tissue at the same location of a scan plane. The two simultaneously imaged tissues produce all image that has a combined echo texture.
Artifacts related to acoustic properties of the tissue or medium
Speed propagation artifact occurs because sound propagates through different body tissues or media at different velocities. Sound travels more slowly through soft tissue than fluid, and even more slowly through fat. As a result, sound that must traverse fatty tissue completes its round trip back to the transducer in a longer period of time. However, to produce an image, US equipment assumes an average tissue velocity of 1540 cm/s. Based on round-trip time and inaccurate depth assignment is made to reflectors posterior to tissue such as fat, fluid and cartilage which have acoustical velocities significantly different from the average.
Through-transmission artifacts occur when sound travels through a medium with low attenuation properties before reaching the imaged object. Scanning through fluid will cause objects to be more echogenic than usual. This is particularly relevant or EUS when cysts or peritoneal fluid are encountered around an area of disease.
Artifacts related to the interaction of sound and tissueRefraction occurs when the sound beam at an oblique angle of incidence strikes a boundary between two media which conduct sound at different velocities. The transmitted part of the sound wave can be bent. The proportion of refraction and reflection that occurs depends on the angle of incidence. Complete bending of the beam can occur when the beam is parallel to the interface, obscuring the image of tissue posterior to the interface. Depending on the direction of the sound beam through the two adjacent media, sound waves will converge or diverge,
Posterior shadowing artifacts occur when sound wave penetration of a structure is very limited or absent. When impedance of two tissues differs to Such a degree that a near complete reflection of the Sound beam is produced, a dark shadow is noted where there are no echoes posterior to the interface. Shadowing also occurs at two apposed transitional zones in the same tissue. Refraction can also cause shadowing from the edge of a curved object, typically at the interface of calcium/soft tissue or gas/fluid.
Enhancement artifacts are due to contrasting acoustic impedance in adjacent structures. Posterior enhancement is a deceptive brightness behind a dark area. The higher amplitude of these echoes compared to those of equivalent adjacent tissue arises posterior to a contrastingly low attenuating area, such as a fluid structure. This sound transmission artifact can be used to distinguish cystic from solid structures. Edge enhancement is an infrequently seen artifact which can occur due to refraction at a curved edge. Focal enhancement can occur in the focal region of the transducer.
Phase cancellation occurs when reflected wave segments are out of phase and cancel each other in summation. When a coherent sound wave crosses boundaries between media that conduct sound at different velocities, the adjacent waveforms become distorted and out of phase. The destructive interference produces an area of cancellation resulting in a black streak or absence of signal on the screen.
Attenuation is a loss of sound wave intensity as a consequence of the behaviours of sound as it interacts with matter. The greatest loss is due to absorption. The higher the frequency, the greater the absorption of sound energy. The degree of attenuation is related to wave frequency and the tissue type and shape. Hypoechoic regions, which could be misinterpreted as masses, can occur behind certain tissues that significantly attenuate the sound beam. Because of the high frequency used with EUS, attenuation artifacts must always be considered. Accuracy in imaging the deep parts of soft tissue structures can be limited by shadows produced when a sound beam does not traverse the structure.
Anisotrophy artifacts result from changes in tissue plane orientation relative to the main second beam axis due to the modulating shape of an imaged organ. Tissue echogenicity changes as sound waves are transmitted at different angles through the same structures due to alterations of sound wave behaviour at the tissue reflectors.
Perivascular colour artifact occurs when intravascular blood flow becomes turbulent resulting in vibrations within disturbed tissue surrounding the blood vessel. When the tissue is examined with colour Doppler, the motion of this vibration will be colour-encoded producing a sonographic equivalent of a soft tissue thrill.
Artifacts and errors related to characteristics of tissue
The composition of different tissues and organs determines their ultrasound image characteristics. Acoustic impedance of soft tissues can differ by as Much as 22%. The presence and uniformity of distribution of different tissue components in a soft tissue structure alters its US image. Changes in tissue density and uniformity due to disease can also alter sound wave/tissue interactions. Inherent complexity of tissue properties will be the major consideration for in vitro studies. For in vivo, and human studies, knowledge of anatomical relationships is also critical to proper EUS interpretation.
Artifacts and errors of EUS technique
Important sources of artifacts and errors that arise from improper instrument placement are non-perpendicular scanning, object compression, insufficient contact at the appropriate anatomical acoustic window, and misinterpretation of anatomy. When the incident sound wave encounters a reflector at an angle, the reflected wave will emerge at an equal angle. Part of the incident wave may not reflect, but may be transmitted into the next tissue or medium. The angle of scanning will affect the amplitude of reflected sonographic echoes and the corresponding degree of refraction. Transducer position can affect these properties of sound waves and can produce artifacts.
The most important source of EUS transducer positioning artifact is oblique or tangential scanning, which can result in the appearance of pseudotumours or indistinct margins between parenchyma and vessels through acoustic artifacts such as reverberation, shadowing and enhancement. However, the most common distortion of non-perpendicular scanning is the appearance of a widening or thickening of the layers of the intestinal wall. The best measurements are obtained with the transducer at right angles to the target so that the sound beam is perpendicular to any boundaries between two tissues or media. In performing EUS, one is always trying to optimize image clarity by balancing the highest frequency to obtain the best spatial resolution with a frequency that penetrates deep enough to view the target area. The higher the frequency, the less divergence occurs, and the easier it is to focus the narrowed beam.
The amount of water placed in the balloon surrounding the transducer affects the way the image is generated and can alter the size of the field of optimal focus. Changing the amount of water in the balloon can also be used to move the focal point. However, changing the angle of the echoendoscope and the amount of water in the balloon can easily compress structures so that distortion occurs. Superficial layers of the wall are more sensitive to this distortion than other parts of the intestinal tract or surrounding structures.
Repeat scanning for the same as well as different angles during introduction and withdrawal of the instrument while varying the focal length with the balloon will limit misinterpretation of an EUS image.
The effects of artifacts can be minimized by scanning from different angles, using the narrowest sound beam possible, focusing properly on the target area, avoiding scanning at edges of objects, recognizing secondary images, and adjusting equipment settings (usually the gain). Misinterpretation of anatomy often results from improper transducer location and/or orientation. Misinterpretation also results if surrounding anatomy and structures of the area being scanned are not considered. This is particularly true at the distal oesophagus where the diaphragm complicates interpretation, especially if a hiatal hernia is present.
Application to animal studies
Animal studies have not been as numerous as human studies. Since future improvements of the clinical application of EUS will depend on advances in image interpretation, useful information could potentially be gained through animal studies. Models could be developed to improve correlations of sonographic findings to histology. The investigation of various agents which enhance or change sound wave characteristics of tissues will assist in differentiating normal and pathological states.
In vitro studies
The study of GI tissue from surgical or autopsy material has resulted in many important findings. Correlation of the EUS wall structure to histology (Fig. 13.1) is the most significant.
48-53 Accuracy of these studies has depended on use of experimental systems that ensured maximum axial resolution and optimal focusing during sonographic imaging. Generally, specimens are mounted with needles into a container constructed to permit accurate spatial localization of the tissue and corresponding ultrasound image. Tissue are immersed in different media including water, deaerated water, saline, water soluble gels and fat emulsions. The acoustic coupling properties of these media have not been studied systematically. Fresh specimens as well as tissue fixed with different agents have been studied. Microdissection techniques are generally utilized.Lymph nodes have also been studied with in vitro models.
29,54,55 In addition, contrast agents which can enhance grey scale and Doppler signals have been evaluated with various systems. 56-61 These agents include: fluorocarbons, galactose microspheres, oil-water and fat emulsions. They are used for tissue suspension media, for intraluminal media, and for intravenous injection to alter and enhance vessels and lymph node acoustic properties. Substances injected into the GI wall can be located with EUS (Fig. 13. 1 c). With in vitro and in vivo models this technique could be used to investigate disease or the effects of various substances on specific layers of the GI wall. In vivo techniquesSwine, canine and sheep animal models have been used to study EUS (Snady H, unpublished observations).
20-22,42,62-65 Models Studying gastric ulcers and portal hypertension have been reported.63,64 Experimental systems have been used to test new probes.21-26 Human intraoperative studies have also been performed (Snady H, unpublished observations). Recognizing anatomy correctly for proper image orientation is one of the most difficult aspects of EUS. Consequently, animal models are limited in their applicability to humans because animal anatomy is significantly different. This is particularly true for the pancreas. The normal pig pancreas appears as an inhomogenous structure withscattered hyperechoic, and round or linear foci within the parenchyma. The pancreatic duct is not visibile.
62 In contrast, the human pancreas is homogeneous with a visible duct. The pancreatic duct is visible in dogs, but limited surrounding fat and a separate ventral and dorsal pancreas make correlations to human disease difficult (Snady H, unpublished observations). Investigating and formulating an effective diagnostic system for early chronic pancreatitis has been limited by the difficulty of gaining direct one-to-one histological confirmation of EUS findings. Theexquisite detail that EUS produces is likely eventually to make it the gold standard against which other methods are cornpared.
66Results and discussion
Pathology in GI organs can be imaged with EUS using anatomical landmarks for orientation. In patients without gastric surgery, all landmarks can be located in at least 90% of cases. However, scanning all organs completely would take more than an hour. Thus, in any given examination, certain unrelated structures or areas need not be recorded, if the focus is a specific anatomical site.
Table 13.2 - Accuracy (%) of GI tumour staging and resectability with EUS and CT.| EUS | CT | ||
| Oesophagus | T stage | 85 | 60 |
| N stage | 80 | 55 | |
| M stage | 70 | 70 | |
| Resectability | 80 | 55 | |
| Gastric | T stage | 80 | 40 |
| N stage | 75 | 50 | |
| M stage | 75 | 75 | |
| Resectability | 80 | 70 | |
| Pancreas | T stage | 90 | 50 |
| N stage | 75 | 50 | |
| M stage | 65 | 65 | |
| Resectability | 80 | 40 | |
| Biliary System | T stage | 85 | 45 |
| N stage | 60 | 50 | |
| M stage | 85 | 85 | |
| Resectability | 80 | 50 | |
| Rectum | T stage | 85 | 70 |
| N stage | 80 | 55 | |
| M stage | - | 75 | |
Table 13.2 shows the median accuracy of TNM staging and assessment of resectability for EUS compared to CT scan for various major GI neoplasms.
4 Errors in T stage occur because EUS cannot always distinguish between neoplastic tissue and benign inflammation or fibrosis. Because high frequency Sound waves have a penetration depth of only 2-4 cm from the probe, optimal focus of vessels or structures around a tumour larger than 5 cm may not always be achieved, and vessel or organ involvement may be missed.Errors in N stage can occur for similar reasons.
4,29,52,55 A malignant node can appear to have benign characteristics because micrometastases have not yet caused parenchymal changes that can be seen sonographically. Criteria for lymph node boundaries and echogenicity appear to overlap less than those for size and shape (Table 13.3). Most metastatic lymph nodes are <10mm. By relying almost exclusively on size to evaluate N stage, CT has been insensitive for neoplastic regional lymph nodes. In contrast, because most lymph nodes >10 mm are usually neoplastic, specificity is good when CT is positive. Unlike CT scan, size resolution is not a limitation for EUS. Lymph nodes >3 mm can be found easily. However, even though EUS is superior to other imaging methods in differentiating malignant from inflammatory benign lyph nodes, criteria (Table 13.3) overlap and still require improvement.In a prospective series of 1000 patients where EUS was performed in an office setting, the major complication rate was 0.2% (Snady H, unpublished observations, 1996). Two perforations occurred in patients with oesophageal tumours, one related to preEUS dilation of the tumour. Transient laryngospasm occurred in a third patient. In a worldwide retrospective survey of 42,105 patients,
67 the "major complication rate was also reported to be low (0.05%). Two-thirds of upper GI EUS complications occurred in patients with oesophageal strictures. In 10 of 13 perforations, oesophageal dilation had been performed immediately prior to EUS. Mortality within 30 days of EUS occurred in only I of 42,105 patients surveyed, and was related to one such perforation. Therefore, aggressive dilation of all oesophageal stricture at the time of EUS is not recommended. Table 13.3 - Criteria to differentiate malignant and inflammatory lymph nodes.| Malignant | Benign | |
| Boundries | Sharp | Indistinct |
| Echogenicity * | Echo-poor Homogeneous | Echo-rich Non-homogeneous |
| Shape | Round | Irregular |
| Size | >10mm | <5mm |
EUS provides detailed images of the GI tract. Clinical applications continue to expand. Proper use of equipment and understanding sound wave properties will minimize errors of the method. Appreciation of how artifacts produce certain changes in tissues improves the operator's facility not only to distinguish the true image, but also to use the artifact to interpret pathology and make a diagnosis. Shadowing and enhancement artifacts are frequently useful in this regard. Section thickness and reverberation artifacts are generally more difficult to interpret and use constructively. The sonographer can be seriously misled by artifacts, which if properly recognized can be used to reveal and inform.
Improvements in correlations of sonographic findings to histology will continue to improve with further studies. Development of various agents to enhance or change sound wave characteristics of tissues and differentiate normal and pathological states will become valuable. Further human and animal studies will continue to establish and clarify parameters that will decrease interobserver variability.
36-38,47 The role of EUS in selection of appropriate treatment will depend upon alternatives available for amelioration of symptoms and improvement of quality of life, survival and outcome.4,14 Utility of EUS will continue to increase with application of major advances in ultrasound technology.68Acknowledgement
The author is grateful to Laurel Kiefer for editorial and graphic assistance.
References1.
Wild JJ, Reid JM. Diagnostic use of ultrasound. BrJ Phys Med 1956; 19: 248-257.2.
Lutz H,Rosch W. Transgastroscopic ultrasonography. Endoscopy 1976; 8: 203-205.3.
Sasai T. Development of ultrasonic endoscope. In: Kawai K (Ed) Endoscopic Ultrasonography in Gastroenterology Igaku-Shoin Ltd, Tokyo, 1988, pp. 18-34.4.
Snady H. The role of endoscopic ultrasonography in diagnosis, staging and outcomes of gastrointestinal disease. Gastroenterologist 1994; 10: 91 -110.5.
Snady H. Technical and interpretive pitfalls in initial experience with endoscopic ultrasonography for upper gastrointestinal disease. Lessons learned. Gastroenterology 989; 96: A480.6.
Rosch T. Endoscopic ultrasonography artifacts and problems of interpretation. Gastrointest Endosc 1996; 43: S10-S12.7.
Tytgat G NJ, Tio TL (Eds). Endoscopic Ultrasonography: Proceedings of the 4th International Symposium on Endoscopic Ultrasonography. Scand J Gastroenterol 1986; 21(suppl 123): 1-172.8.
Kawai K (Ed). Endoscopic Ultrasonograpliy in Gastroenterology Igaku-Shoin, Tokyo, 1988.9.
Snady H. Endoscopic ultrasonography: an effective new tool for diagnosing gastrointestinal tumors. Oncology 1992; 6: 63-74.10.
Rosch T, Classen M. Gastroenterological Endosonography (Textbook and Atlas). Thieme Medical Publishers, New York, 1992.11.
Lightdale C (Ed). Endoscopic ultrasonography. In: Sivak M Jr (Ed) Gastrointest Endosc Clinics N Am 1992; 2: 557-749.12.
Rosch T (Ed). Endoscopic ultrasonography: State of the art - 1995. Part 1. In: Sivak M Jr (Ed) Gastrointest Endosc Clinics N Am 1995; 5: 475-698.13.
Rosch T (Ed) Endoscopic ultrasonography: State of the art - 1995. Part II. In: Sivak M Jr (Ed) Gastrointest Endosc Clinics N Am 1995; 5: 699-898.14.
Gillard V, Mainguet P, Vicari F, Florent Ch (Eds). 3rd Endoscopic Ultrasonography Belgian Meeting. Acta Endoscopia 1995; 25: 407-556.15.
Erickson R, Chang K. Normal anatomy, part 1. In: Training for Endosonography - An Interactive Learning Tool. CD-ROM, Olympus America and Astra Merck Inc, 1996.16.
Caletti G, Gerrari A, Barbara L. Normal endosonographic anatomy of the esophagus and stomach. In: Lightdale C (Ed) Endoscopic ultrasonography. Gastrointest Endosc Clinics N Am 1992; 2: 601-614.17.
Snady H. Encloscopic ultrasonography images of the normal retroperitoneum. In: Lightdale C (Ed) Endoscopic ultrasonography. Gastrointest Endosc Clinics N Am 1992; 2: 637-655.18.
Dancygier H. Endosonographic evaluation of biliary tract disease. In: Lightdale C (Ed) Endoscopic ultrasonography. Gastrointest Endosc Clinics N Am 1992; 2: 697-714.19.
Wiersema MJ, Hawes RJ. Normal colorectal anatomy and benign colon lesions. In: Lightdale C (Ed) Endoscopic ultrasonography. Gastrointest Endosc Clinics N Am 1992; 2: 715-728.20.
Vilmann P. Endoscopic ultrasonography-guided fine needle aspiration biopsy of lymph nodes. Gastrointest Endosc 1996; 43: S24-S29.21.
Silverstain FE, Martin RW, Kimmey MB, Jiranek GC, Francklin DW, Proctor A. Experimental evaluation of an endoscopic ultrasound probe: in vitro and in vivo canine studies. Gastroenterology 1989; 96: 1058-1062.22.
Taniguchi D, Martin R, Trowsers E, Silverstein F. Simultaneous M-mode echoesophagram and manometry in sheep esophagus. Gastrointest Endosc 1995; 41: 582-586.23.
Gress F, Park K, Sangvi N, Kopecky K, Hawes R. A comparison study of high frequency ultrasound imaging of the canine GI tract at 20, 30 and 50 MHz frequencies. Gastrointest Endosc 1996; 43: s54.24.
Takemoto T, Yanai H, Tada M et al. Application of ultrasonic probes prior to endoscopic resection of early gastric cancer. Endoscopy 1992; 24(suppl 1): 329-333.25.
Bartram CI. Anal sphincter disorders. Gastrointest Endosc 1996; 43: S32-S34.26.
Yasdua K. Ultrasonic probes For pancreaticobiliarv strictures. Gastrointest Endosc 1996; 43: S35-S37.27.
Odegaard S, Kimmey M. Location of the Muscularis mucosa on high frequency gastrointestinal ultrasound images. Eur J Ultrasound 1994; 1: 39-50.28.
Wiersema M, Wiersema L. High-resolution 25 Megahertz ultrasonography of the gastrointestinal wall: histologic correlates. Gastrointest Endosc 1993; 39: 499-504.29.
Tio TL, Tytgat GNJ. Endoscopic ultrasonography in analyzing per-intestinal lymph node abnormality. Preliminary results in vivo and in vitro. Scand J Gastroenterol 1986; 21(suppl 123): 158-163.30.
Behahrs 0, Henson D, Hutter R (Eds). Manual for Staging of Cancer, 3rd edn. American joint Committee on Cancer. JB Lippincott Co, Philadelphia, 1988.31.
Hermanek P, Sobin L (Eds). International Union Against Cancer (UICC): TNM classification of malignant tumours 4th edn. Springer-Verlag, Berlin, 1987.32.
Denoix PF, Six annees d'enquete permanente cancer. Bull Inst Natl Hyg 1944; 1: 1-69.33.
Denoix PF. De L’importance d'une nomenclature unifiee dans l'etude du cancer. Rev. Med Franc 1947; 36: 1124-1128.34.
Hutter RV. At last - Worldwide agreement on the staging of cancer. Arch Surg 1987; 122: 1235-1239.35.
Tio L. The TNM staging system. Gastrointest Endosc 1996; 43: S 19-24.36.
Snady H, Bruckner H, Siegel J, Cooperman A, Neff R, Kiefer L. Endoscopic ultrasonographic criteria of vascular invasion by potentially resectable pancreatic tumors. Gastrointest Endosc 1994; 40: 326-333.37.
Palazzo L, Burtin P. Interobserver variation in tumor staging. In: Rosch T (Ed) Endoscopic Ultrasonography: State of the art - 1995. Part 1. In Sivak M Jr (Ed) Gastrointest Endosc Clinics N Am 1995; 5: 559-568.38.
Roubein L. Inter-observer variability in endoscopic ultrasonography: a prospective study. Acta Eitdoscopica 1995; 25: 549-550.39.
Caletti GC, Brocchi E, Ferrari A et al. Guillotine needle biopsy as a supplement to endosonography in the diagnosis of gastric submucosal tumors. Endoscopy 1991; 23: 251-254.40.
Wiersema M, Hawes R, Tao L-C et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc 1992; 38: 35-39.41. Snady H. Combined endoscopic ultrasound and guillotine needle biopsy in the diagnosis of Submucosal tumors of the gastrointestinal tract (Abstract). Am J Gastroenterol 1993; 88: 1599.
42.
Kimmey M, Martin R. Fundamentals of endosonography. In: Lightdale C (Ed) Endoscopic ultrasonography. Gastrointest Endosc Clinics N Am 1992; 2: 557-573.43.
Kremkau F, Taylor K. Artifacts in ultrasound imaging. J Ultrasound Med 1986; 5: 227-237.44.
Scanlan KA. Sonographic artifacts and their origins. Am J Radiol 1991; 156: 1267-1272.45.
Rubin J, Adler R, Bude R, Fowlkes J, Carson P. Clean and dirty shadowing at US: a reappraisal. Radiology 1991; 181: 231-236.46.
Kliewer MA, Hertzberg BX, George PY, McDonald JW, Bowie JD, Carroll BA. Acoustic shadowing from uterine leiomyomas: sonographic-pathologic correlation. Radiology 1995; 196: 99-102.47 Catalano M. Normal structures on endoscopic ultrasonography: visualization measurement data an interobserver variation. In: Rosch T (Ed) Endoscopic ultrasonography: State of the art - 1995. Part 1. In: Sivak M Jr (Ed) Gastrointest Endosc Clin N Am 1995; 5: 474-486.
48.
Kimmey MB, Martin RW, Haggitt RC, Wang Y, Franklin DW, Silverstein FE. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology 1989; 96: 433-441.49.
Aibe T, Fuji T, Okita K, Takemoto T. A fundamental study of the normal layer structure of the gastrointestinal wall visualized by endoscopic ultrasonography. Scand J Gastroenterol 1986; 21(supp123): 6-15.50.
Tio TL, Tytgat GNJ. Endoscopic ultrasonography of normal and pathologic upper gastrointestinal wall structure. Scand J Gastroenterol 1986; 21(suppl 123):27-33.51.
Boscaini M, Montori A. Transrectal ultrasonography: Interpretation of normal intestinal wall structure for the preoperative staging of rectal cancer. Scand J Gastroenterol 1986; 21(suppl 123): 87-98.52.
Bolondi L, Caletti G, Casanova P, Villanacci V, Grigioni W, Labo G. Problems and variations in the interpretation of the ultrasound feature of the normal upper and lower GI tract wall. Scand J Gastroenterol 1986; 21(suppl 123): 16-26.53.
Silverstein F, Kimmey M, Martin R et al. Ultrasound and the intestinal wall: Experimental methods. Scand J Gastroenterol 1986; 21(suppl 123): 34-40.54.
Heinz A, Mildenberger P, Georg M, Garcia A, Junginger Th. In vitro studies of lymph node analysis. In: Rosch T (Ed) Endoscopic ultrasonography: State of the art - 1995. Part 1. Gastrointest Endosc Clin N Am 1995; 5: 577-586.55. Aibe T, Ito T, Yoshida T et al. Endoscopic ultrasonography of lymph nodes surrounding the upper GI tract. Scand J Gastroenterol 1986; 21(suppl 123): 164-169.
56. Bhutani M, Hoffman B, Van Velse A, Hawes R. SHU508A (galactose microparticles) as contrast agent during endoscopic ultrasound. Gastrointest Endosc 1996; 43: A416.
57. Andre M, Nelson T, Mattrey R. Physical and acoustical properties of perfluoroocytlromide, an ultrasound contrast agent. Invest Radiol 1990; 25: 983-987.
58. Mattrey R. The potential clinical impact of perflubron emulsion on general sonography. Invest Radiol 1991; 26: S186-187.
59.
Mattrey RF, Strich G, Shelton RE et al. Perfluorochemicals as US contrast agents for tumor imaging and hepatosplenography: preliminary clinical results. Radiology 1987; 163: 339-343.60.
Fritzsch T, Hilmann J, Kampfe M, Muller N, Schobel C, Siegert J. SHU508, a transpulmonary echocontrast agent: initial experience. Invest Radiol 1990; 25: S160-S161.61.
Gallez B, Demeure R, Debuyst R et al. Evaluation of nonionic niroxyl lipids as potential organ specific contrast agents for magnetic resonance imaging. Mag Res Imag 1992; 10: 445-455.62.
Bhutani MS, Hoffman BJ, Hawes RH. A swine model for endosonographic pancreatic imaging (Abstract). Gastrointest Endosc 1996; 43: s53.63. Maruoka A, Fujishima H, Misawa T, Chijiiwa Y, Nawata H. Evaluation of acetic acid-induced gastric ulcers in dogs by endoscopic ultrasonography. Scand J Gastroenterol 1993; 28: 1055-1061.
64. Jutabha R, Jensen D, Machicado, G, Hirabayashi K. Reliability of endoscopic ultrasound probe imaging of canine abdominal veins before and after sclerotherapy in a blinded study. Gastrointest Endosc 1996; 43: A297.
65.
Taniguchi D, Martin R, Trowers E, Silverstein F. Simultaneous M-mode echoesophagram. and manometry in sheep esophagus. Gastrointest Endosc 1995; 41: 582-586.66.
Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol 1986; 21(suppl 123): 123-129.67. Rosch T, Dittler HK, Fockens P, Yasuda K, Lightdate C. Major complications of endoscopic ultrasonography: results of a survey of 42,105 cases (Abstract). Gastrointest Endosc 1993; 39: 370.
68. Wayt Gibbs W. Ultrasound's new phase. Sci Am 1996; 274: 32-34.